Pharmaceutical Jurisprudence MCQs with Answers for GPAT / University examination
1. For manufacturing blood products or to operate blood bank licence is issued in the form no:
A. 28A
B. 28
C. 28 B
D. 28C
2. The term of patent for ordinary invention from the date of patent is:
A. 7 years
B. 14years
C. 5 years
D. 10years
3. An example of artificial colour is:
A. Titanium dioxide
B. Caramel
C. Cochineal
D. Curcumin
4. All adults employed for work in a shop or other establishment should not be works daily for more than:
A. 10Hours
B. 9 Hours
C. 11 Hours
D. 12Hours
5. Import of drug for personal use contains average doses in mg up to:
A. 200
B. 150
C. 100
D. 50
6. All finished alcoholic preparations should be stored in jars or bottle each containing not less than
A. 1 litre
B. 2litres
C. 2.25 litres
D. 5 litres
7. Every year the register of State pharmacy council is required to print the registers:
A. 1 st January
B. 1 st March
C. 1 st April
D. 1 st June
8. The pharmacy council of India is also known as:
A. Central council of pharmacy
B. Central druggist association
C. State pharmacy council
D. Joint Pharmacy Council
9. The period in hours of training to be undertaken by a student pharmacist in a hospital is:
A. 500
B. 750
C. 600
D. 800
10. Education regulations are approved by:
A. State government
B. Central government
C. Pharmacy council of India
D. Education institutions
11. Manufacture without bond licences are issued by:
A. Excise commissioner
B. Drug inspector
C. Government Analyst
D. Registrar
12. The committee that advises the D.T.A.B and various government is:
A. D.C.C.
B. D.E.C
C. CCUM
D. P.C.I
13. Full form of FSSAI is:
A. Food standard and safety authority of India.
B. Food safety and standard administrator of India
C. Food safety and standard affiliated institute
D. Food safety and standard authority of India
14. Standards for Cosmetics belongs to
A. Schedule A
B. Schedule C
C. Schedule S
D. Schedule X
15. Alcoholic preparation can be stored in a warehouse for a maximum period of:
A. 7 years
B. 5 years
C. 3 years
D. 1year
16. The Drug price control order came into force in the year:
A. 1970
B. 1987
C. 1955
D. 1960
17. Government opium factory is situated at :
A. Ghazipur
B. Lucknow
C. Srinagar
D. Calcutta
18. The first edition of Indian Pharmacopoeia was published in the year:
A. 1940
B. 1985
C. 1955
D. 1950
19. Life period of drugs is dealt in
A. Schedule ‘Q’
B. Schedule ‘R’
C. Schedule ‘P’
D. Schedule ‘T’
20. In which year the D.E.C Committee is established and submitted report respectively:
A. 1920,1921
B. 1931,1930
C. 1940,1945
D. 1930.1931
21. The central Drug laboratory is established at:
A. Calcutta
B. Lucknow
C. Mumbai
D. Kasauli
22. The members of the D.T.A.B hold the office for a period of:
A. 1 Year
B. 3 Years
C. 5 years
D. 7 Years
23. Which of the following are prohibited to be imported:
A. Toilet Preparations
B. Misbranded drugs
C. Unani drugs
D. Schedule ‘C’
24. Offences and penalties under NDPS for Opium poppy:
A. NLT 10 years which may extend to 20 years and with fine NLT 1lakh rupees which may extend to 2 Lakh rupees.
B. NLT 5 years which may extend to 10 years and with fine NLT 10 lakh rupees which may extend to 20 Lakh rupees.
C. NLT 3 years which may extend to 6 years and with fine NLT 1 lakh rupees which may extend to 2 Lakh rupees.
D. NLT 15 years which may extend to 20 years and with fine NLT 1 lakh rupees
which may extend to 2 Lakh rupees.
25. Advertisement of Prescription drugs are meant for:
A. Physician
B. Manufacturer
C. Patient
D. Retailer
26. Biological and microbiological tests are conducted at:
A. Mumbai
B. Calcutta
C. Chennai
D. Kasauli
27. DTAB contains _________ Ex-Officio members
A. Five
B. Six
C. Four
D. Eight
28. Post marketing surveillance comes under clinical trial
A. Phase-I
B. Phase-II
C. Phase-III
D. Phase-V
29. In 1985, one of the following Act was passed:
A. Narcotic and psychotropic substance act
B. Drug and magic remedies act
C. The medical termination of pregnancy act
D. Poisonous Act
30. Grant of licence to manufacture a drug requires
A. Form 24
B. Form 25
C. Form 26
D. Form 27
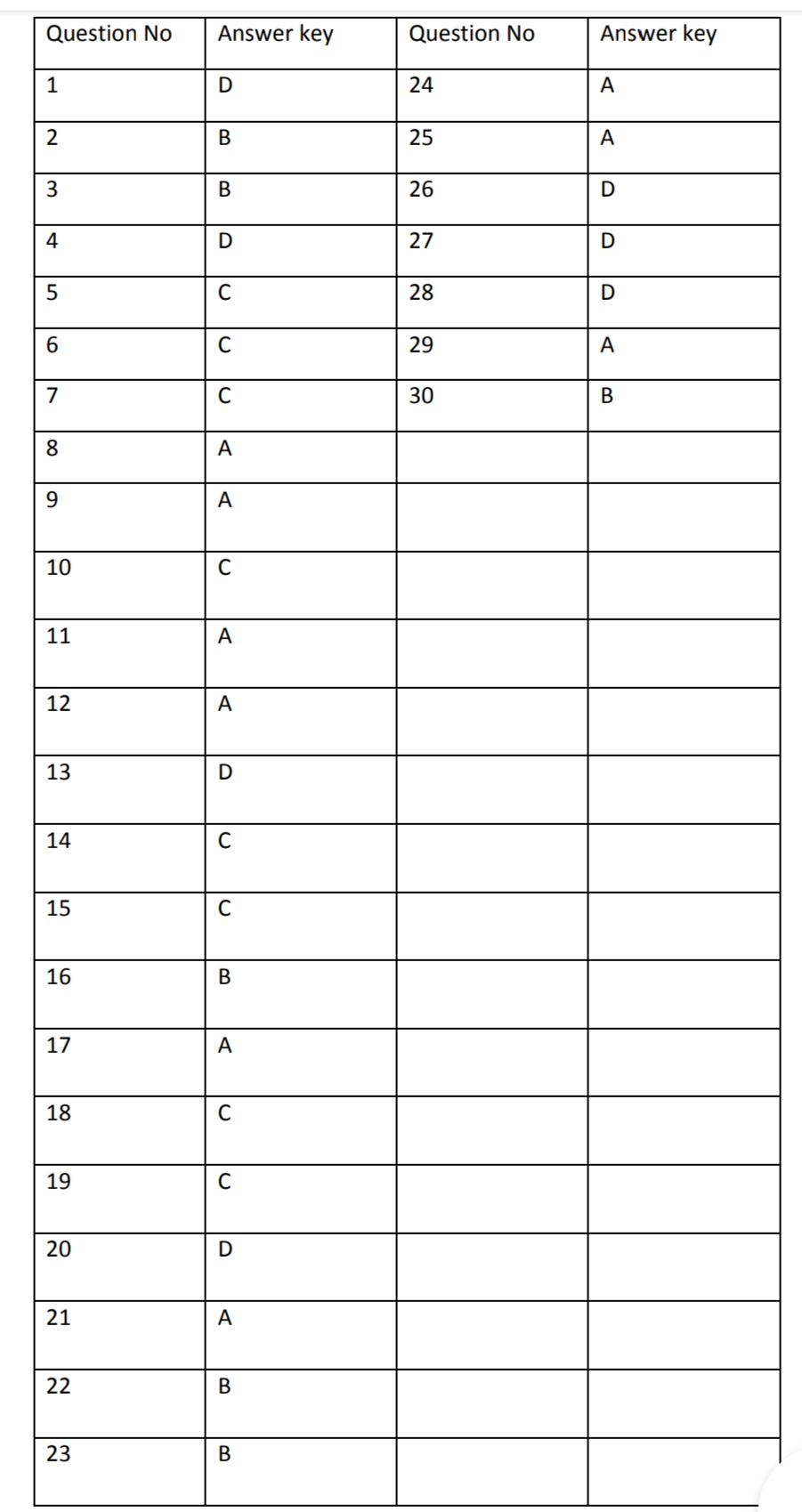
Answers