PHARMACEUTICS
51. Water soluble bases are also known as
(a) Greasy ointment bases
(b) Greaseless ointment bases
(c) Both
(d) None
52. In pastes, the concentration of insoluble powder substances in
(a) 20%-50%
(b) 50%-100%
(c) 50%-75%
(d) None
53. Jellies are generally
(a) Water-soluble bases
(b) Water-insoluble bases
(c) Both
(d) None
54. As per USP XX, the term “object-ionable” means
(a) An organism can cause disease or the presence may interrupt the function of the drug or lead to deterioration of the product
(b) Pathogens if they produce disease or infection, in the newborn or debilitated persons
(c) Organisms or their toxins that are responsible for human disease or infection
(d) None
55. The success or failure of a preservative in protecting a formulation against microbial spoilage depends on
(a) Interaction between preservative with surfactant
(b) Interaction between preservative with active substances
(c) Sorption by packaging materials
(d) All the above
56. A suppository is generally intended for use in
(a) Rectum
(b) Vagina
(c) Urethra
(d) All the above
57. Vaginal suppositories also called as
(a) Pessaries
(b) Simple suppositories
(c) Bougies
(d) None
58. “Oleum theobromae” was first recommended by
(a) A.B. Taylor
(b) Griffin
(c) Stocks’s
(d) None
59. Weight of rectal suppository for adults?
(a) 1 g
(b) 2 g
(c) 5 g
(d) None
60. Weight of rectal suppository for children is
(a) 1 g
(b) 2 g
(c) 5 g
(d) None
61. Urethral suppositories also called as
(a) Pessaries
(b) Bougies
(c) Both
(d) None
62. Urethral suppositories having which shape
(a) Oviform shape
(b) Torpedo shape
(c) Pencil shape
(d) None
63. Weight of urethral suppository for males & females respectively
(a) 4 & 2
(b) 2 & 4
(c) 4 & 6
(d) 6 & 4
64. Shape of vaginal suppositories is
(a) Oviform shape
(b) Torpedo shape
(c) Pencil shape
(d) None
65. Rectal suppositories mainly used for the treatment of
(a) Constipation
(b) Hemorrhoids
(c) Both
(d) None
66. The number of milligrams of KOH required neutralizing free acids & saponify the esters contained in 1 g of fat is known as
(a) Iodine value
(b) Saponification value
(c) Water number
(d) Acid value
67. The number of grams of iodine that reacts with 100 g of fat is known as
(a) Iodine value
(b) Saponification value
(c) Water number
(d) Acid value
68. The number of milligrams of KOH required neutralizing free acids in 1 g of fat is known as
(a) Iodine value
(b) Saponification value
(c) Hydroxil value
(d) Acid value
69. The number of milligrams of KOH required neutralize the acetic acid used to acetylate 1 g of fat is known as
(a) Iodine value
(b) Saponification value
(c) Hydroxil value
(d) Acid value
70. Which of the following method is used to manufacture suppositories
(a) Hand molding
(b) Compression molding
(c) Pour molding
(d) All the above
71. Which of the following is most commonly used suppository base
(a) Cocoa butter
(b) PEG 1000
(c) PEG + Hexanetriol
(d) None
72. Cocoa butter available in which forms?
(a) α-form
(b) β-form
(c) γ-form
(d) All
73. The solidification point of cocoa butter lies between
(a) 12 – 13o
(b) 20 – 30o
(c) 5 – 10o
(d) None
74. Which of the following method is simple & oldest method of preparation of suppositories?
(a) Hand molding
(b) Compression molding
(c) Pour molding
(d) All the above
75. Most commonly used method for producing suppositories on both a small & large scale is
(a) Hand molding
(b) Compression molding
(c) Pour molding
(d) All the above
76. Which formula can be used to calculate the amount of base that is replaced by active ingredients?
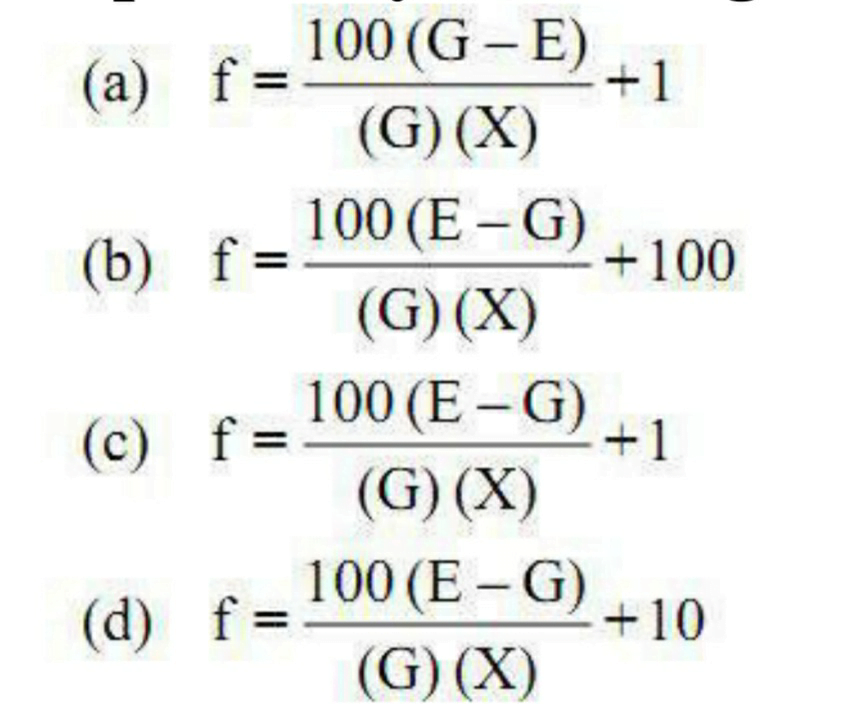
Correct Answer:- C
77. Rancidity generally results from
(a) Auto oxidation
(b) Decomposition of unsaturated fats
(c) Both
(d) None
78. Which of the following is not antioxidant?
(a) BHT
(b) BHA
(c) Tocopherol
(d) Theobroma oil
79. Suppositories are generally evaluated by
(a) Melting range test
(b) Breaking test
(c) Liquefaction
(d) All the above
80. Which of the following materials are used in pharmaceutical packaging?
(a) Glass
(b) Plastic
(c) Metal
(d) All the above
81. Which of the following packaging material is protect the drug content against light
(a) Plastic containers
(b) Amber colored glass containers
(c) Both
(d) None
82. Major disadvantages of glass as a packing material are
(a) Fragility
(b) Weight
(c) Both
(d) None
83. Composition of glass is
(a) Sand
(b) Soda ash
(c) Lime stone & Cullet
(d) All the above
84. Soda ash also known as
(a) Pure silica
(b) Sodium carbonate
(c) Lime stone
(d) Calcium carbonate
85. Which of the following one is a broken glass & acts as fusion agent
(a) Cullet
(b) Soda ash
(c) Lime stone
(d) Sand
86. Which of the following methods are used in the production of glass
(a) Blowing
(b) Drawing
(c) Pressing & casting
(d) All the above
87. To produce molten glass, which of the following method is used
(a) Blowing
(b) Drawing
(c) Pressing
(d) Casting
88. To protect the contents of a bottle from the effects of sunlight by UV rays, which glass is used?
(a) Amber glass
(b) Red glass
(c) Both
(d) None
89. To evaluate the chemical resistance of glass, which of the following tests are conducted?
(a) Powder glass
(b) Water attack test
(c) Both
(d) None
90. Which of the following test is performed on crushed grains, to evaluate the chemical resistance of glass?
(a) Powder glass
(b) Water attack test
(c) Both
(d) None
91. Which of the following test is performed on whole container?
(a) Powder glass
(b) Water attack test
(c) Both
(d) None
92. Type I glass is also known as
(a) Borosilicate glass
(b) Regular soda-lime glass
(c) Treated soda-lime glass
(d) None
93. The advantages of plastic containers over glass containers are
(a) Easy formation
(b) Resistance to breakage
(c) Freedom of design
(d) All the above
94. Plastic containers are generally made from the following material
(a) Polyethylene
(b) Polypropylene
(c) Polystyrene
(d) All the above
95. Which of the following ingredients are present in rubber stopper?
(a) Vulcanizing agent
(b) Softner
(c) Antioxidant
(d) All the above
96. Which of the following packaging systems are identified by the FDA?
(a) Blister pack
(b) Strip pack
(c) Bubble pack
(d) All the above
97. Which of the following packaging is commonly used for packaging of tablets & capsules?
(a) Blister pack
(b) Strip pack
(c) Both
(d) None
98. Which of the following materials offer moisture barrier properties?
(a) Aclar
(b) Cellophane
(c) Polyester
(d) All the above
99. Which of the following mechanism is responsible for release of encapsulated core materials?
(a) By disrupting the coating by pressure
(b) By offering permeability facilities
(c) By leaching of permanent fluid
(d) All the above
100. Preformulation studies mainly focus on
(a) Physical properties of new compound
(b) Chemical properties of new compound
(c) Physico-chemical properties of new compound
(d) None
