Contents
INTRODUCTION
HISTORY
OPIOID RECEPTORS
SAR OF MORPHINE ANALOGUES
OPIOID AGONISTS
I) Morphine analogues
II) Synthetic compounds unrelated to morphine
MIXED OPIOID AGONIST/ANTAGONISTS AND PARTIAL AGONISTS
OPIOID ANTAGONISTS
INTRODUCTION
• Opioid analgesics were earlier termed as narcotic analgesics; but this term is lesser in use nowadays, because several narcotic analgesics do not cause narcosis (sleep or loss of consciousness) and many drugs which cause narcosis are not analgesics.
• The term narcotics, these days, is reserved only for those drugs whose manufacture, distribution and prescribing comes under Narcotic Drug Act.
HISTORY
• The pharmacist Surturner isolated an alkaloid from opium in 1803 and named morphine.
• Until the 1980s, the term “opiate” was used to describe any natural or synthetic
compound that was structurally related to morphine.
• In the mid-1970s, it was discovered that peptides in the brain showed similar
pharmacological actions to morphine, but were structurally not related to morphine. This prompted a change in the word from “opiate” to “opioid”.
• Today, opium alkaloids, their synthetic derivatives and synthetic peptides with morphine like pharmacological effects are called as opioids.
OPIOID RECEPTORS
• Morphine and other opioids show their actions by interacting with three opioid receptors – μ (mu), κ (kappa) and δ (delta) as either agonists, partial agonists or competitive antagonists.
• Morphine and other pure agonists have greatest affinity for μ receptors but have lower affinity for κ and δ receptors.
• Various pharmacological actions at opioid receptors is described in following table.

SAR OF MORPHINE ANALOGUES
• Morphine is the prototypical opioid with selective agonistic activity on μ opioid receptors.

• The structure of morphine is composed of five fused rings – A, B, C, D and E ring.
• It has five chiral centres with absolute stereochemistry 5(R), 6(S), 9(R), 13(S) and 14(R). The naturally occurring isomer of morphine is levo-rotatory.
• The A ring and the basic nitrogen (which exists predominantly in the protonated form at physiological pH) are the two most common structural features found in opioid agonists.
• A tertiary amine with methyl substituent is necessary for good opioid activity.
• Increasing the size of N-substituent to 3 to 5 carbons (especially with unsaturation or small carbocyclic ring) results in compounds that are antagonists at some or all opioid receptor types.
• Further increasing the size of N-substituent returns agonistic activity. E.g., N-phenylethyl substituted opioid is 10-fold more potent as a μ agonist than the N-methyl analogue.
• Replacement of C3-OH by hydrogen results in 10 times decrease in activity.
• Replacement of C3-OH by -OCH3 group results in codeine, which itself is a relatively weak μ agonist, but it undergoes slow metabolic O-demethylation to give morphine, which accounts for much of its morphine like action.

• Replacement of C3-OH by -OCOCH3 group (esterification / acetylation) results in decrease
in activity, while same replacement at C6 results in increase in activity.
• The 3,6-diacetyl derivative of morphine is known as heroin.

• Heroin’s high lipophilicity compared to morphine results in enhanced penetration through the blood-brain barrier. Once in the body, plasma and tissue esterases hydrolyse the 3- acetyl group to produce 6-acetylmorphine, which has increased μ agonistic activity than morphine.
• The 6-keto-7,8-dihydro derivative of morphine is known as hydromorphone, which is 8 to 10 times more potent than morphine. Similarly 6-keto-7,8-dihydro derivative of codeine (hydrocodone) is more active than codeine.
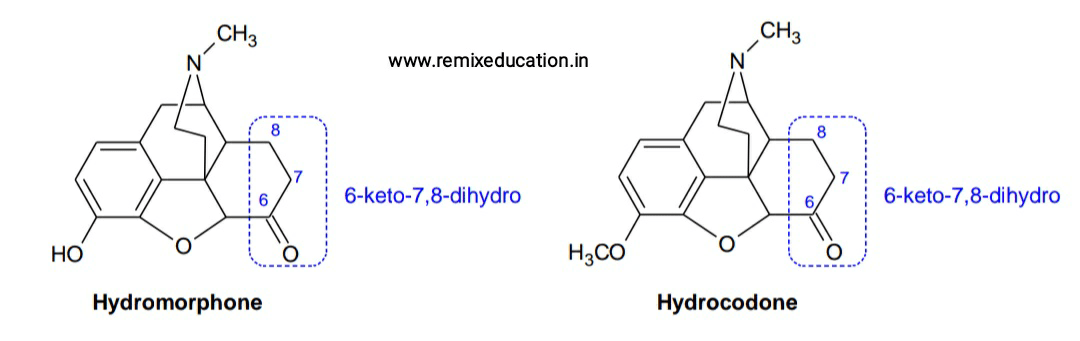
• The introduction of β-OH group at C14 enhances μ agonistic activity and decreases antitussive activity. E.g., oxymorphone and oxycodone

• Only the levo-isomer of morphinans possess opioid activity, while dextro isomer possess antitussive activity.
• Levorphanol and butorphanol are the two morphinan derivatives. Levorphanol is approximately 8 times more potent than morphine while butorphanol is μ antagonist and κ agonist.
• Removal of C ring from morphinans results in benzomorphans.

• Pentazocine is the benzomorphan derivative. It is a weak antagonist at μ receptors.
• The opioid activity of A- and D- ring analogues of morphine (4-phenylpiperidine class) was discovered. Meperidine, the first agent of this class, is one-fourth active than morphine as μ agonist.

OPIOID AGONISTS
I) Morphine analogues
(‒)-Morphine

• It is a μ agonist, used for severe, acute and chronic pain.
• It is three to six times more potent when given intramuscularly than when given orally. This is due to first pass 3-O-glucuronidation of morphine to give inactive metabolite.

• It is a weak μ agonist, used for moderate to mild pain.
• Its 10% of an oral dose is metabolized to morphine, which contributes significantly to its analgesic effect.
• It is more selective cough suppressant.
(‒)-Hydromorphone and (‒)-Oxymorphone

• Both are 8 to 10 time more potent μ agonist than morphine and used for severe pain. (‒)-Hydrocodone and (‒)-Oxycodone

• Both are equipotent μ agonist as morphine, used orally for moderate pain.

• It is morphinan derivative, approximately 8 times more potent μ agonist than morphine.
• Its clearance half-life is more than its duration of hours. This leads to a build-up of the drug in the body and results in excessive sedation.
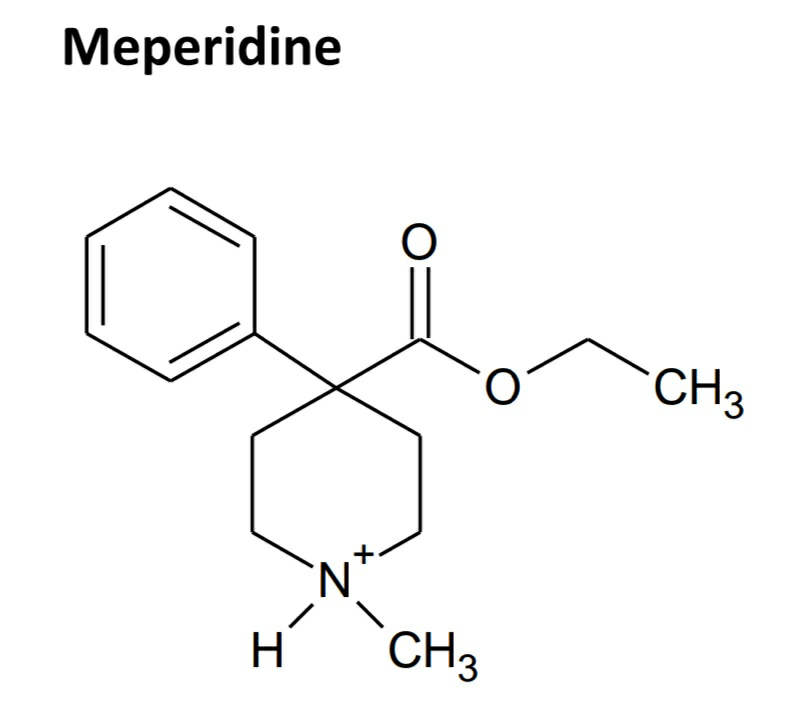
• It is a μ agonist with approximately one-tenth the potency of morphine after intramuscular dose and one-fourth the potency of morphine after oral dose.
• Prolonged dosage of meperidine may cause an accumulation of the metabolite normeperidine, which has only weak analgesic activity, but it causes CNS excitation and can initiate grand mal seizures.
II) Synthetic compounds unrelated to morphine
(±)-Tramadol

• The (+)-isomer exhibits opioid analgesic activity, while (‒)-isomer inhibits reuptake of noradrenaline and 5-HT, and thus activates monoaminergic spinal inhibition of pain.
• It is non-addicting and does not cause respiratory depression or constipation.

• It is equipotent μ agonist as morphine.
• It is used as a racemic mixture, but nearly all of the activity resides with R-(‒)-isomer.
• It has longer duration of action compared to most other μ agonists upon oral dosage.
Synthesis of Methadone


Dextropropoxyphene ((+)-propoxyphene)
• It is a weak μ agonist (approximately one-twelfth potency of morphine), used either as a single agent or in combination with NSAIDs for mild to moderate pain.

• It is 4-anilidopiperidine, which is discovered by structural modification from 4- phenylpiperidine class.
• It is approximately 80 times more potent μ agonist than morphine.
• Because of its shorter duration of action, it has been used in combination with nitrous oxide for anaesthesia.

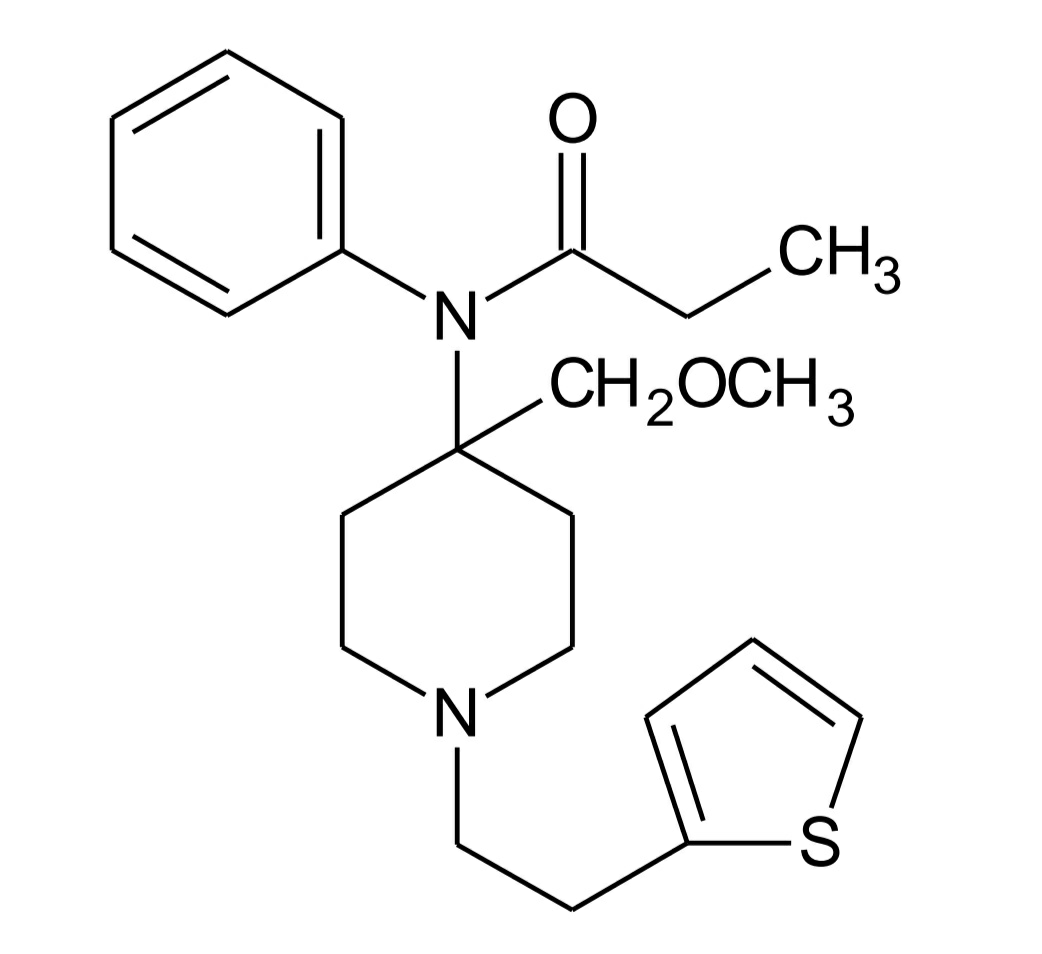
Sufentanil
• Addition of methoxymethyl group at 4th position of piperidine ring and bioisosteric replacement of phenyl ring by thiophene in fentanyl results in sufentanil, which is 600 to 800 times more potent μ agonist than morphine.
• It has a rapid onset and shorter duration of action than fentanyl and is used for anaesthesia.

Alfentanil
• Replacement of thiphene ring by tetrazole ring in sufentanil results in decrease in potency and decrease in ionization.
• Being more unionized, alfentanil penetrates the blood-brain barrier faster than other fentanyl derivatives and has a faster onset and shorter duration of action.
• It is used for anaesthesia.
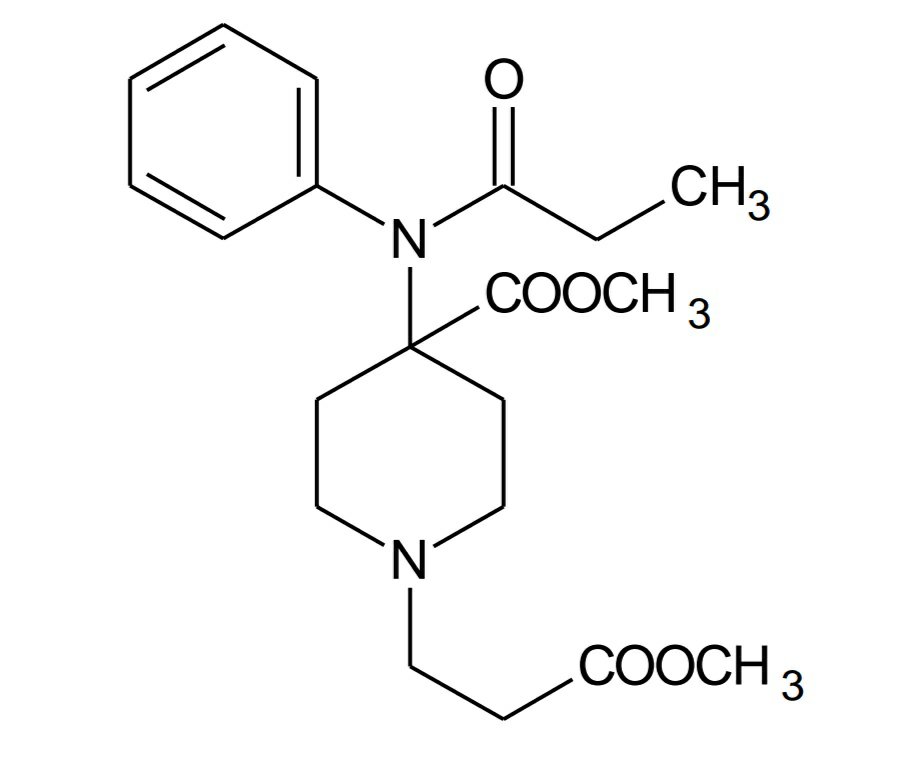
Remifentanil
• It is 15 to 20 times more potent μ agonist than alfentanil.
• It has a very short duration of action due to the presence of hydrolysable ester groups.
• It is used for analgesia in combination with injectable anaesthetic agents.
MIXED OPIOID AGONIST/ANTAGONISTS AND PARTIAL AGONISTS

(‒)-Buprenorphine
• It is a morphinan derivative.
• It is an agonist at κ receptors and an antagonist at μ receptors.
• Because of μ antagonistic property, it will immediately induce abstinence syndrome if given to a person addicted to μ agonist.

(‒)-Nalbuphine
• It is a morphinan derivative.
• It is an agonist at κ receptors and an antagonist at μ receptors.

(‒)-Pentazocine
• It is a benzomorphan derivative.
• It is an agonist at κ receptors and a weak antagonist at μ receptors.
OPIOID ANTAGONISTS

Naloxone
• It is the drug of choice for morphine poisoning and for reversing neonatal asphyxia due to opioid use during labour.
• It is also used to treat overdose with other opioids.

Naltrexone
• It is 3 to 5 times more potent than naloxone.
• It differs from naloxone in being orally active and having a longer duration of action which makes it suitable for ‘opioid blockade’ therapy of postaddicts.
