Chlorpromazine Hydrochloride
Structure –
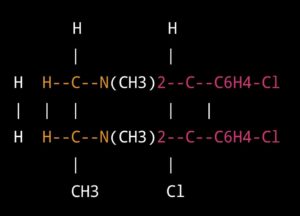
it is a crystalline powder that is a derivative of phenothiazine. Its molecular formula is C17H19ClN2S·HCl, and its chemical structure is:
This molecule has several functional groups, including a chloro group (Cl) attached to the nitrogen atom (N), a sulfur atom (S) attached to a phenyl ring, and a secondary amine group (NH) attached to the phenyl ring. The hydrochloride salt of chlorpromazine is added to increase its solubility in water.
Synthesis –
The synthesis of it involves several steps. Here is a simplified overview of the process:
- Starting material: 2-chloroacetophenone
- 2-chloroacetophenone is reacted with ethylenediamine to form 2-chloro-N-(2-diethylaminoethyl)acetamide.
- The product from step 2 is then reacted with sulfur and hydrochloric acid to form 2-chloro-N-(2-diethylaminoethyl)thioacetamide.
- This thioacetamide is then oxidized to form 2-chloro-N-(2-diethylaminoethyl)thioacetamide oxide.
- The oxide is then reacted with hydrogen chloride gas
to form chlorpromazine hydrochloride.
The overall reaction can be summarized as follows:
2-chloroacetophenone + ethylenediamine + sulfur + hy
drochloric acid + hydrogen chloride gas → chlorpromazine hydrochloride
The resulting it is then purified through recrystallization to obtain a pure crystalline product.
SAR –
The Structure-Activity Relationship (SAR) of Chlorpromazine Hydrochloride can be summarized as follows:
- Phenothiazine nucleus: Chlorpromazine Hydrochloride belongs to the phenothiazine group of antipsychotic drugs. The phenothiazine nucleus plays a significant role in its antipsychotic activity.
- Alkyl side chain: Chlorpromazine Hydrochloride has an alkyl side chain at position 2 of the phenothiazine ring. This side chain length and structure affects the potency and pharmacokinetic properties of the drug. Longer side chains increase potency, but also increase toxicity and reduce solubility.
- Substituents on the side chain: The presence of a tertiary amino group at position 3 on the side chain is important for antipsychotic activity. The presence of a chloro group at position 2 on the side chain increases the potency of Chlorpromazine Hydrochloride.
- Ring substitution: Substitution on the phenyl ring at positions 3 and 7 with a halogen or methyl group increases potency, while substitution with bulky groups decreases potency.
- Hydrogen bonding: it can form hydrogen bonds with receptors in the brain, which contribute to its antipsychotic activity.
Overall, the SAR of Chlorpromazine Hydrochloride suggests that the phenothiazine nucleus and alkyl side chain play a significant role in its antipsychotic activity, while the substitution on the phenyl ring and hydrogen bonding contribute to its potency and pharmacological properties.
Mechanism –
- The exact mechanism of action of it is not fully understood, but it is believed to act primarily as a dopamine receptor antagonist. It binds to and blocks dopamine receptors in the brain, particularly the D2 receptors, which reduces the activity of dopamine signaling pathways. This leads to a decrease in psychotic symptoms, such as hallucinations and delusions.
- In addition to its dopamine receptor antagonism, Chlorpromazine Hydrochloride also has affinity for other receptors in the brain, including serotonin receptors, alpha-adrenergic receptors, and histamine receptors. Its antagonism of these receptors may contribute to its sedative and anticholinergic effects.
- it also has an effect on the reuptake of norepinephrine and serotonin, two neurotransmitters involved in mood regulation. It inhibits the reuptake of these neurotransmitters, which can lead to an increase in their activity.
- Overall, Chlorpromazine Hydrochloride’s mechanism of action is complex and involves interactions with multiple receptors and neurotransmitter systems in the brain. Its primary effect as a dopamine receptor antagonist is believed to be responsible for its antipsychotic activity, while its effects on other receptors and neurotransmitter systems contribute to its side effects and therapeutic properties.
Uses –
Chlorpromazine hydrochloride is a medication used primarily to treat mental health conditions such as schizophrenia and other psychotic disorders. Some of the uses of chlorpromazine hydrochloride are:
- Schizophrenia: Chlorpromazine hydrochloride is an effective treatment for schizophrenia. It can reduce hallucinations, delusions, and disordered thinking.
- Bipolar disorder: Chlorpromazine hydrochloride can be used in combination with other medications to treat bipolar disorder.
- Nausea and vomiting: Chlorpromazine hydrochloride can be used to treat nausea and vomiting caused by cancer chemotherapy, radiation therapy, or surgery.
- Anxiety: Chlorpromazine hydrochloride can help alleviate symptoms of anxiety, including agitation and restlessness.
- Agitation: Chlorpromazine hydrochloride can be used to calm patients who are agitated or aggressive.
- Tourette syndrome: it can be used to treat the tics and other symptoms associated with Tourette syndrome.
- Migraine headaches: it can be used to treat severe migraines that do not respond to other treatments.
It is important to note that chlorpromazine hydrochloride can have serious side effects, and should only be used under the guidance and supervision of a healthcare professional.