Evaluation of parenteral
- Sterility testing:
It is done to determine that particular lot of material is sterile.
Principle:
It is based on principle that if bacteria or fungi are kept in a medium which provide favourable conditions
for growth, the organism will grow and their presence can be detected.
Steps in sterility testing: - Selection of sample size
- Selection of amount of product to be tested
- Method of testing
- Observation and results
- Selection of sample size (containers):
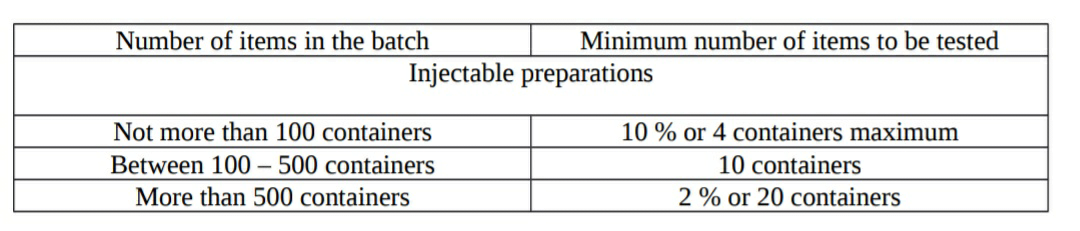
- Selection of amount of product to be tested:
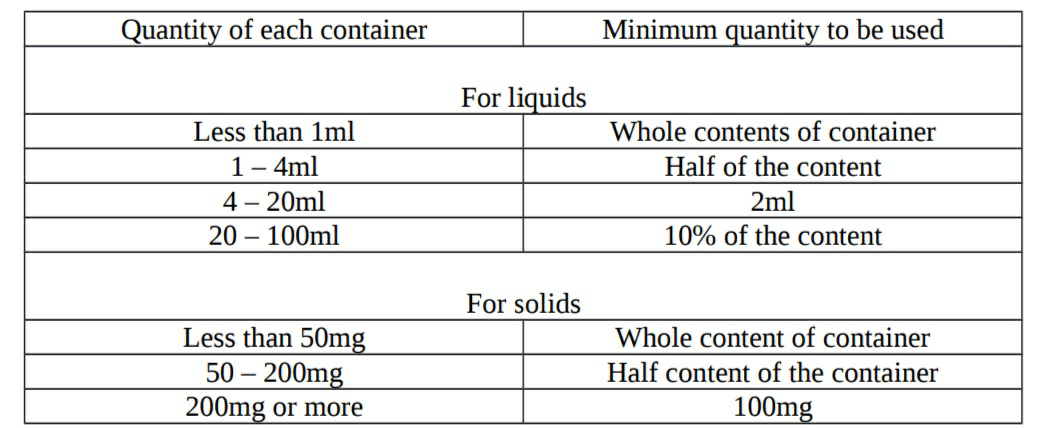
Methods of testing:
- Membrane filtration method
- Direct inoculation method
Membrane filtration method:
Sample is filtered through a membrane filter of 0.45µm size
Then membrane is divided into two halves
The first half is placed in 100ml of soyabean- digest media for growth of fungi at 20°c – 25°c for 7days.
Other half is placed in 100ml of fluid thioglycollate media for bacterial growth at 30°c – 35°c for 7 days
Then observe the growth
Direct inoculation method:
In this method, specified amount of sample is aseptically removed from the container
Transferred it into the culture media
Mix the sample with media and incubate for 14 days
Observe the growth
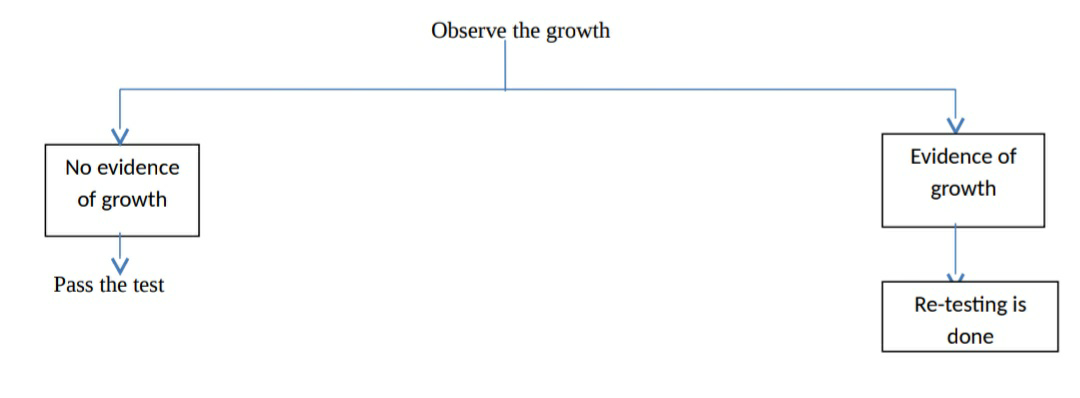
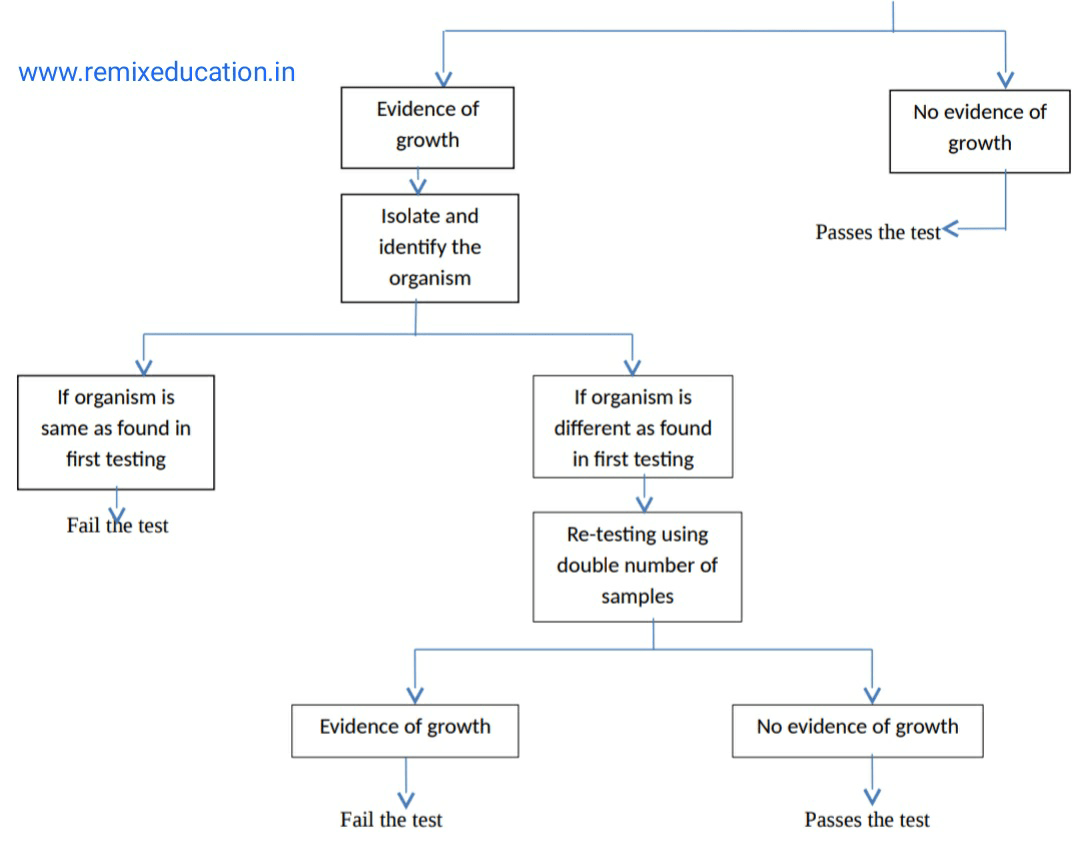
Pyrogen testing:
Pyrogens are the endotoxins of the gram negative bacteria, thermostable, soluble in water, polysaccharides, pass through bacteria proof filters and cause rise in body temperature of human. There are two methods for Pyrogen Testing:
- In-vivo testing (Rabbit test)
- In-vitro testing (LAL test)
- Rabbit test:
- Principle:
It involves the measurement of rise in body temperature of rabbit when a IV injection of sample to be tested is injected.
Material required:
• Thermometer, glass wares, syringes, needles, retaining boxes for rabbits.
• Three healthy adult rabbits of same sex.
Following rabbits should not use:
• A rabbit having weight less than 1.5kg
• A rabbit having temperature more than 39.8°c
• A rabbit showing temperature variation more than 0.2°c between two readings.
Method:
• The test is performed in AC room.
• The sample to be tested is dissolved in pyrogen free saline solution.
• Warm the sample solution to 38.5°c before injection.
• The volume to be injected is not less than 0.5ml or not more than 10ml per kg body weight
• Thermometer is inserted into the rectum of the rabbit
• Two normal temperature readings are taken an interval of 30 minutes before injecting the sample Solution
• Mean of two reading is calculated and noted as initial reading
• The sample solution is injected into the ear vein of rabbit in doseppof 0.5 – 10ml/kg.
• Record the temperature of each rabbit after 30 minutes of injecting the sample solution
• Record the temperature of each rabbit upto 3 hours an interval of 30 minutes.
• The difference between the initial temperature and maximum temperature recorded after injection is noted as response
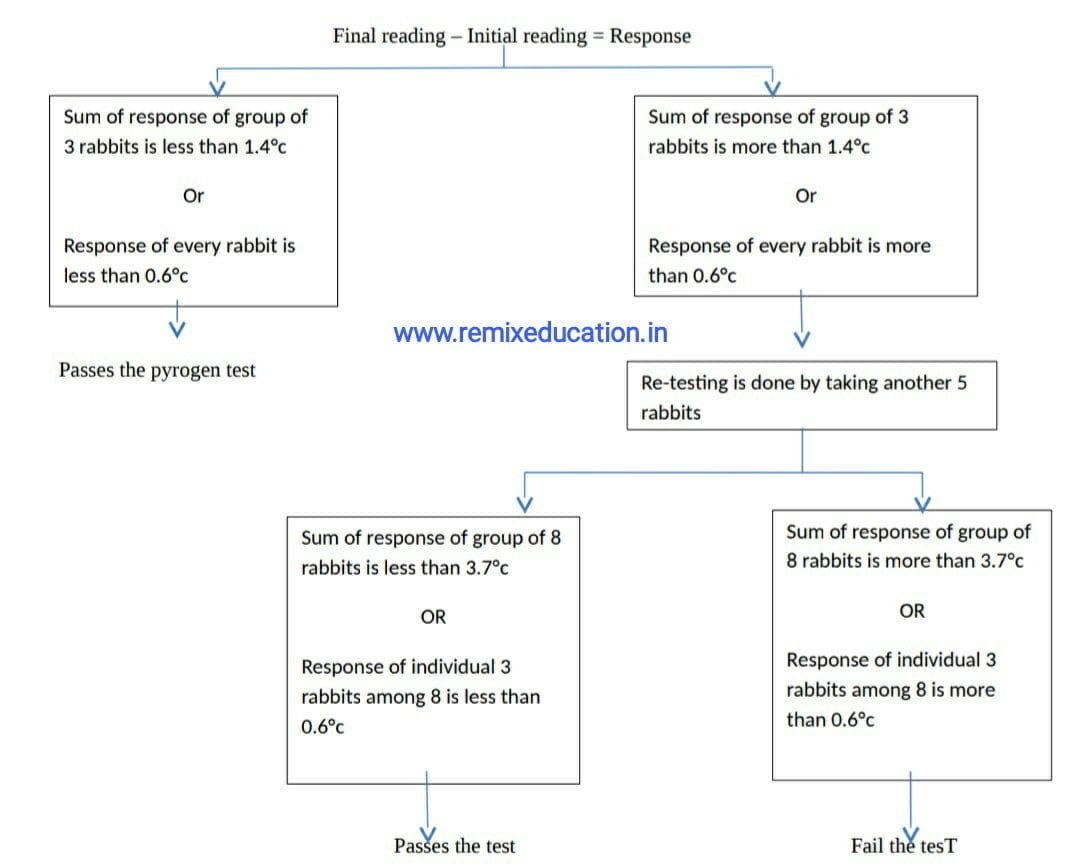
Interpretation:
In- vitro test (LAL Test):
Principle:
It involves the use of LAL reagent (from horse shoe crab) which shows coagulation in presence of polysaccharides and pyrogens are also lipopolysaccharides. If pyrogens will be present it will show coagulation.
Method:
• 0.1ml sample is with 0.1ml LAL reagent in a depyrogenated glass plate.
• Plate is incubated at 37°c for 1 hour.
• Observe the plate under microscope.
• Presence of coagulation shows presence of pyrogens.
• If coagulation not occur it means pyrogens are absent.
Leakage testing:
It is done to check that the container in which parenteral preparation is filled is hermatically sealed.
Method:
• To check leakage, the container is immersed in 1% methylene blue solution in a vacuum chamber under negative pressure and vacuum is released.
• If colored solution entered into the container, it shows presence of leakage into the container.
Clarity testing:
It is done to check the presence of any foreign particle or impurity in the container.
Method:
• To perform the clarity test, an instrument having black and white background and light source is used.
• Dark colored preparations are seen against white background and light colored preparations are seen against dark background.
• If any impurity or particle is present it will detected visually and preparation is rejected.
