Physical Pharmacy I MCQs Questions & Answer Key
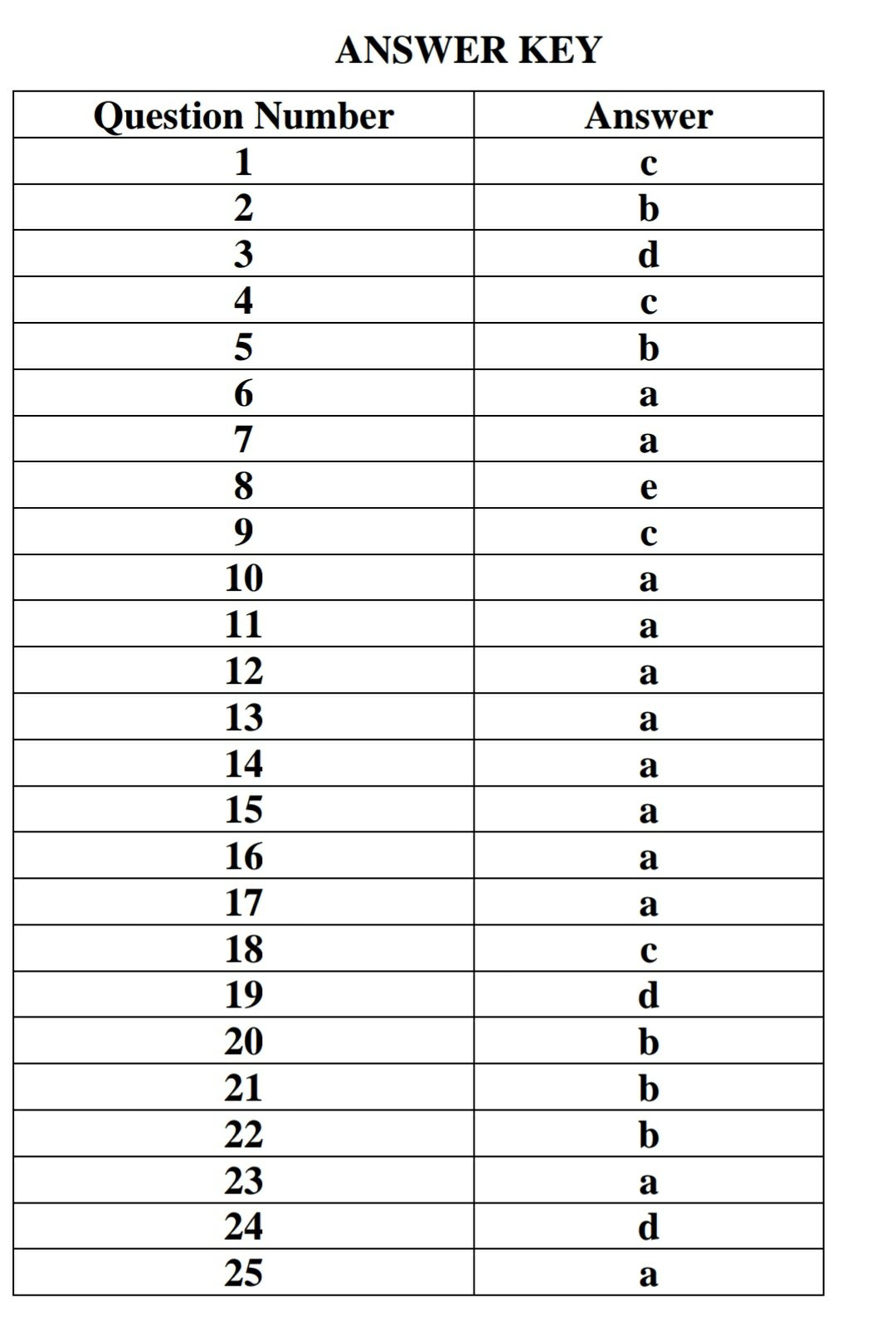
1.If 25 g of a liquid occupies 20 cm3 in a measuring cylinder, what is the density of the liquid?
a) 0.25 g cm-3
b) 0.8 g cm-3
c) 1.25 g cm-3
d) 5 g cm-3
2.Which property measures the resistance of a liquid to flow?
a) Density
b) Viscosity
c) Volume
d) Solubility
3.What is the concentration of a 0.5 % (w/v) solution when expressed as mg mL-1?
a) 0.005 mg mL-1
b) 0.05 mg mL-1
c) 0.5 mg mL-1
d) 5 mg mL-1
4.Which of the following excipients may be used to limit the presence of microorganisms in a liquid formulation?
a) Purified water
b) Sodium lauryl sulphate
c) Benzalkonium chloride
d) Ascorbic acid
5.What is the role of xanthan gum within some liquid formulations?
a) Regulate pH
b) Control viscosity
c) Enhance solubility
d) Enhance stability
6.Which of the following liquid dosage forms requires a sterile formulation?
a) Eye drops
b) Spray applied to skin
c) Shampoo
d) Oral syrup
7.In term pH, H indicates
a) Hydrogen
b) Haemoglobin
c) Helium
d) Half life
8. For an aqueous solution of hydrochloric acid (HCl) the pH was found to be 4.18 units. What is the concentration of HCl in this solution?
a) 2.2 x10-3 M
b) 1.4 x 10-3 M
c) 3.2 x 10-4 M
d) 4.8 x 10-4 M
e) 6.6 x 10-5 M
9. In which method, tonicity is calculated by adding water to the drugs to make an isotonic solution
a. Sodium chloride equivalent method
b. Cryoscopic method
c. White Vincent Method
d. Potentiometric method
10. The tonicity of solutions can be determined by
a. Colorimetric method
b. pH method
c. Dew point method
d. Viscosity
11. .Cryoscopic method for adjusting tonicity and pH comes under
a. Class I Method
b. Class II Method
c. Class III Method
d. Class IV Method
12. The solution having an osmotic pressure greater than that of 0.9% w/v sodium chloride is called
a. Hypertonic solutions
b Isoosmotic solution
c. Hypotonic solution
d. Isotonic solution
13. The value 14 on pH scale indicates
a. Strongly alkaline
b. Neutral
c. Strongly acidic
d. Weakly acidic
14. . Which of the following methods are used to measure pH value?
a. pH paper
b. Cloudmethod
c. Raft methhod
d. White Vincent Method
15.. The term pH was first used by
a. Soren Peter Lauritz Sorensen.
b. Louis Pasteur
c. James Kelvin
d. Alfard Columb
16. Maximum buffer capacity occur when
a. pH = pKa
b. pH > pKa
c. pH< pKa
d. pKa=pKb
17. Which of the following Colligative property
a) osmotic pressure
b) solubility solute
c) dissociation of solute
d) Hydroxyl ion concentration
18. Colligative property of solution is related to
a) pH of the solution
b) pKa
c) Total numbers of solute particle in solution.
d) total numbers of ions in the solution
19. pH of pharmaceutical buffer can be calculated by
a) pH partition theory
b) Michalis Menten equation
c) Noys Whiteny equation
d) Handerson Hasselbalch equation
20. Cryoscopy method used for calculation of isotonic solution is based upon
a) Molecular concentration of drug
b) Freezing point depression of drug
c) Boiling point elevation of drug
d) pH of the solution
21. Buffer solutions
a) are strongly acidic
b) resist pH change
c) decrease the pH of the solution
d) increase the pH of the solution
22. Apparatus used to determine surface tension of liquid is
a. Capillary tube viscometer
b. Du Nouy tensiometer
c. Rotometer
d. Rheometer
23. HLB Scale was introduced by
a. Griffin
b. Brunauer
c. Emmett
d. Teller
24. Surfactant with HLB value more than 16 indicate
a) Wetting agent
b) Detergent
c) Spreading agent
d) Solubilizing agent
25. The different between work of adhesion and work of cohesion is called
a) spreading coefficient
b) surface tension
c) interfacial tension
d) viscosity
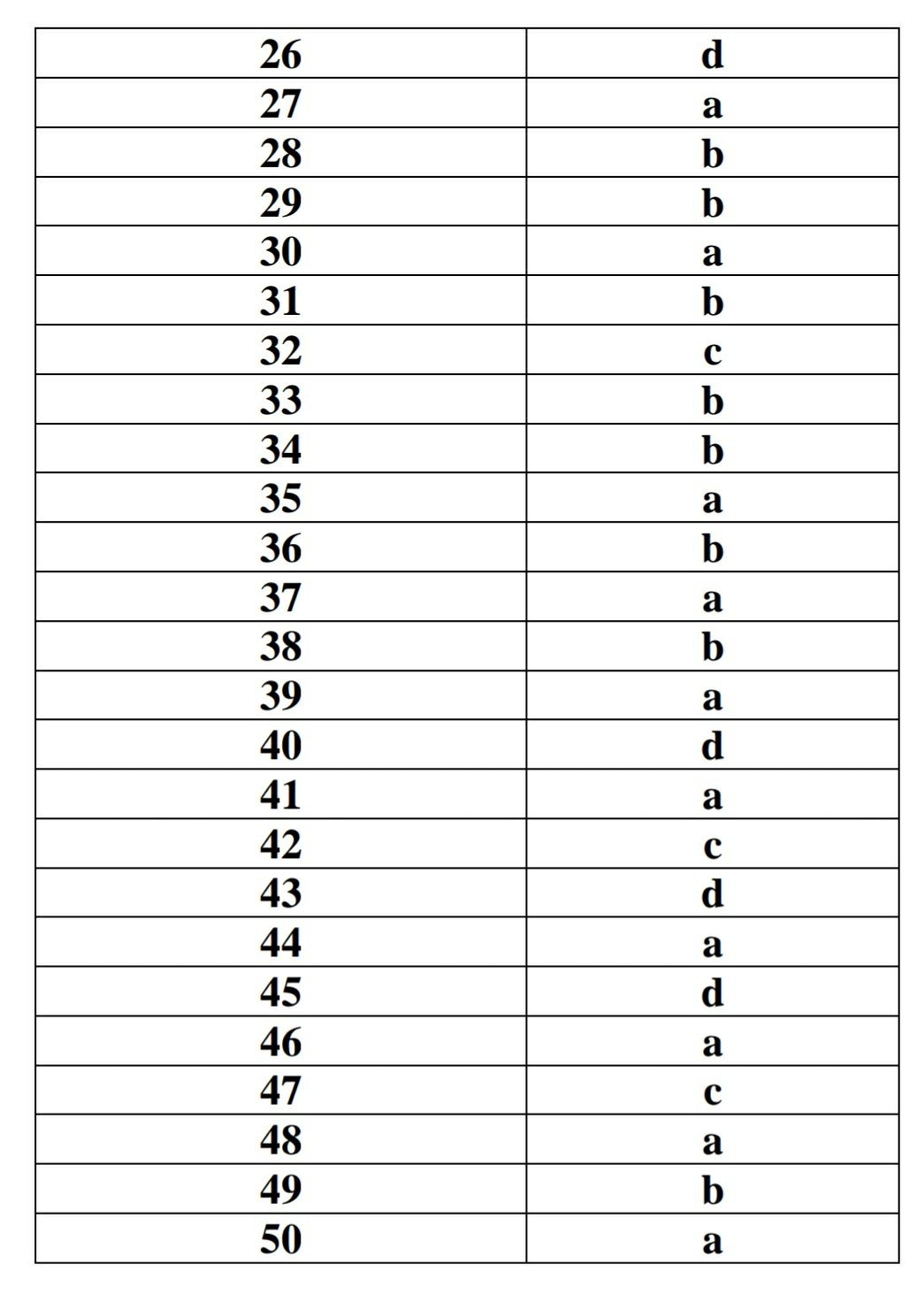
26. The unit of surface tension is
a) N/m2
b) dyne/m
c) N/cm
d) N/m
27. SLS is example of
a) Anionic surfactnat
b) Non ionic surfactant
c) Cationic surfactant
d) Amphiphilic
28. Stalagmometer is used to determine
a) Viscosity
b) Surface Tension
c) Solubility
d) Particle size
29. Which of the following method used exclusively to determine Interfacial tension.
a) Capillary rise method
b) Du Nouy tensiometer
c) Drop Count method by stalagmometer
d) Drop weight method by stalagmometer.
30. Which of the following method is used to determine surface tension of liquid.
a) Capillary rise method
b) Ostwald Viscometer method
c) Sprowely method
d) Griffin method
31. If the wetting angle between solid and liquid is 180 degree it indicates
a) Good spreading property
b) Poor spreading property
c) Highly soluble in each other
d) Miscible with each other
32. As per Langmuir adsorption isotherm what is equilibrium point.
a) Rate of adsorption is greater than rate of desorption
b) Rate of adsorption is lesser than rate of desorption
c) Rate of adsorption and rate of desorption is equal
d) Rate of adsorption and rate of desorption is zero.
33.Which is more stronger
a) Physical Adsorption
b) Chemical Adsorption
34. Raindrops are spherical in shape because of
a) Capillary
b) Surface Tension
c) Downward motion
d) Acceleration due to gravity
35. If common salt is dissolved in water, then the surface tension of saltwater is
a) Increased
b) Decreased
c) Not changed
d) First increases then decrease
36. A drop of oil is placed on the surface of the water. Which of the following statements is correct?
a) It will remain on it as a sphere
b) It will spread as a thin layer
c) It will partly be as spherical droplets and partly as thin films
d) It will float at the distorted drop on the water surface.
37. How do insects such as pond skaters stay afloat on water?
a) Because of high surface tension of water
b) As they can swim
c) Because they are less dense than water
d) Because they have special legs.
38. The contact angle forming between magnesium stearate and water is larger than that between lactose and water because:
a) Magnesium stearate is more hydrophilic
b) Magnesium stearate is more hydrophobic
c) Lactose has more surface energy
d) Both have equal hydrophilicity
39.. What is the main result of adding surfactants into a liquid composed of two immiscible phases such as oil and water?
a) Reduction in the interfacial tension between the phases
b) Increase in the interfacial tension between the phases
c) Catalysation of a chemical reaction between the phases
d) Nothing happens
40.. A surfactant with a very large Hydrophile-Lipophile Balance (HLB) value (e.g. 40) is expected to function as a:
a) Anti-foaming agent
b) Water in oil (w/o) emulsifier
c) Oil in water (o/w) emulsifier
d) Solubility enhancer
41. Number of moles of a solute per liter of solution
a. Molarity
b. Molality
c. Normality
d. Equivalency
42. Mole fraction of solute is the ratio of
a. the number of moles of solute
b. the total number of moles of solute and solvent
c. the number of moles of solute and the total number of moles of solute and solvent.
d. the number of moles of solvent minus number of moles of solute.
43. Which of the following is not a system of measure of solubility
a. Mass per volume
b. Molarity
c. Milliequivalent
d. Enthalpy
44. According to USP, Sparingly soluble means the Parts of solvent required for one part of solute is
a. 30-100
b. 10-30
c. 100-1000
d. Less than 1
45. The Normaility of a solution depends on the
a. volume of solvant
b. temperature
c. pressure.
d number of dissociable H and OH ions.
46. At a specified temperature, maximal amount of solute that can dissolve in an amount of solvent is known as
a. Solubility
b. Dissolution
c. Diffusion
d. Capacity
47. Additional or Extra solute will not dissolve in a
a. saturated solution
b. dilute solution
c. concentrated solution
d. non aqueous solution
48. Solubility Curve is a curve drawn between
a. solubility and temperature
b. solubility and pressure
c. solubility and mole fraction
d. solubility and enthalpy
49. The solubility of gas___________________ with rising temperature
a. Increase
b. Decrease
b. Remain constant
d nothing Happen
50 The chemical reaction in which the opposite electric charge ions come together in solution and form a distinct chemical entity is called
a. Association
b. Solvation
c. Combination
d. Capacitance