CASE study of asthma patient
A 62 years old man presented with four weeks history of cough which had become productive in the past 2 weeks with night sweats and lethargy. These symptoms has worsened over past weeks. Past medical history revealed that he had Asthma and Type 2 Diabetes. He has recently been prescribed Gliclazide 5mg for DM. As his sugar control didn’t return normal following a coarse of oral corticosteroids prescribed for an acute asthmatic attack. On examination he was thin, feverish and pale. Temperature was 101oF, B.P. was 120/70 mm of Hg and pulse was 80 beats/min. He complained of chest pain which was increased by cough. Medication on which the patient was.
R
Salbutamol 100g 2 puffs SOS
Beclomethasone Dipropionate 100g 2 puffs b.i.d
Gliclazide 5mg 1 tab O.D
Paracetamol 2 tab SOS
Serum urea, Electrolytes, Hb, LFT’s were all normal. B.S.R was 4 mol/L
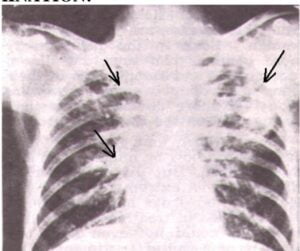
He had expiratory wheeze. Chest X-ray shows bilateral Epical Shadowing, more marked on left lobe. There was an evidence of Calcification on both left and right sides. Blood, Sputum and urine samples were sent to the microbial examination. MANTAUX was ordered. A diagnosis of Pulmonary T.B. was made on the basis of Chest X-Ray.
R
Rifampicin 600mg
INH 300mg 1 time/day
Pyrazinamide 2g
Ethambutol 900mg
+ the usual therapy of Asthma & Diabetes. The patient was feeling fine after two week following therapy and sugar and blood counts were within the normal range. His LFT’s were as follows:
A.L.T. 75 Units/L (Normal: 7-35 Units/L)
A.S.T 80 Units/L (Normal: 7-40 Units/L)
Alk. Phosp. 47 Units/L (Normal: 25-90 Units/L)
Bilirubin 12 mol/L (Normal: > 17mol/L)
Bilirubin Direct 2 mol/L (Normal: > 6mol/L
1. Outline a Pharmaceutical Care Plan.
2. What might have caused abnormal L.F.T’s. What actions do you recommend?
3. What else would you add in therapy?
4. What point would you like to inform to patient?
5. Find out the interactions in the regimen.
1. PHARMACEUTICAL CARE PLAN:
1. PERSONAL INFORMATION:
NAME: AGE: 62 years GENDER: Male
ADDRESS:
2. MEDICAL INFORMATION:
An old age patient with productive cough, night sweats and lethargy. He was taking Anti-asthmatic and Anti-diabetic drugs.
3. MEDICAL HISTORY:
• Past medical history suggests that he had Asthma and Diabetes Type-II.
• Current medical situation reveals that he has cough which became productive in last 2 weeks with night sweats and lethargic condition, with generalized weakness, pale complexion and fever. He also complained for a chest pain which was increased by coughing. He had a poor sugar control for last 2 weaks.
4. MEDICATION HISTORY:
Patient has recently been prescribed for Gliclazide 5mg for DM. But he is also on Oral Corticosteroid therapy for acute asthmatic attack. His past medication record shows:
R
Salbutamol 100g 2 puffs SOS
Beclomethasone Dipropionate 100g 2 puffs b.i.d
Gliclazide 5mg 1 tab O.D
Paracetamol 2 tab SOS
5. PHYSICAL FINDINGS:
Appearance: Pale feverish
Temperature: 101oF
Blood Pressure: 120/70 mm of Hg
Pulse Rate: 80 beats/min
6. LAB RESULTS:
Serum Urea, Electrolytes, Hemoglobin, LFT’s is all normal.
B.S.R was 4 mmol/L.
7. X-RAY EXAMINATION:
The chest X-ray clearly explains the bilateral apical shadowing more marked on left lobe and calcification on both left and right lobe. This X-ray findings and complain of expiratory wheeze is a characteristic feature of Pulmonary Tuberculosis.
8. LAB TEST:
Montaux test was ordered. The Mantoux test (also known as the Mantoux screening test, Tuberculin Sensitivity Test, Pirquet test, or PPD test for Purified Protein Derivative) is a diagnostic tool for tuberculosis. A standard dose of 5 Tuberculin units (0.1 mL) is injected intradermally (into the skin) and read 48 to 72 hours later. A person who has been exposed to the bacteria is expected to mount an immune response in the skin containing the bacterial proteins. The result should be recorded as “0 mm”.
5 mm or more is positive in
• HIV-positive person
• Recent contacts of TB case
• Persons with nodular or fibrotic changes on chest x-ray consistent with old healed TB
• Patients with organ transplants and other immunosuppressed patients
10 mm or more is positive in
• Recent arrivals (less than 5 years) from high-prevalence countries
• Injection drug users
• Residents and employees of high-risk congregate settings (e.g., prisons, nursing homes, hospitals, homeless shelters, etc.)
• Mycobacteriology lab personnel
• Persons with clinical conditions that place them at high risk (e.g., diabetes, prolonged corticosteroid therapy, leukemia, end-stage renal disease, chronic malabsorption syndromes, low body weight, etc)
• Children less than 4 years of age, or children and adolescents exposed to adults in high-risk categories
15 mm or more is positive in
• Persons with no known risk factors for TB
9. PATIENT COMPLAINTS:
Fever, productive cough, wheezing, chest pain, weakness, paleness.
10. CONDITION OR THE PATIENT NEED:
The patient is diagnosed as having Pulmonary T.B and diabetes. The patient was feeling fine after two week following therapy but above LFT’s values clearly indicate a liver abnormality. A.L.T is a specific liver enzyme which shows a marked rise after using corticosteroids and first line T.B. drugs (R.I.P)
11. OUTCOME FOR CONDITION:
The problematic condition should be treated and managed and symptomatic treatment of cough and fever should be done immediately. The body temperature should be at normal range. Respiratory difficulty should be resolved by ventilation.
12. REGIMEN
The patient was on following medication after the confirmation of Pulmonary T.B with diabetes.
R
Rifampicin 600mg
INH 300mg 1 time/day
Pyrazinamide 2g
Ethambutol 900mg
+ The usual therapy of Asthma & Diabetes.
13. EVALUATION:
The patient was feeling fine after two week following therapy and sugar and blood counts were within the normal range. His LFT’s were as follows:
A.L.T. 75 Units/L (Normal: 7-35 Units/L)
A.S.T 80 Units/L (Normal: 7-40 Units/L)
Alk. Phosp. 47 Units/L (Normal: 25-90 Units/L)
Bilirubin 12 mol/L (Normal: > 17mol/L)
Bilirubin Direct 2 mol/L (Normal: > 6mol/L
After the initial treatment for 2 weeks with Anti-T.B drugs, there is a marked rise in Liver enzymes which should be brought to normal. The diabetes may be poorly controlled.
14. PHARMACEUTICAL BASED PROBLEMS:
• The patient may not be properly advised by the prescriber about the rationale of use of oral corticosteroids and there is an improper use of Anti-T.B drugs (Rifampicin, Isoniazid, and Pyrazinamide) in large doses which may cause drug induced hepatitis.
• Elevation of liver enzyme is a clear evidence of liver abnormality which is probably due to use of such drugs because these drugs are metabolized in the liver and are Cyt-P450 inducer. There is an also evidence that concurrent use of these drugs with Acetaminophen may contribute to increased liver toxicity.
15. INTERACTIONS
• Rifampin (Ingredient of rifampin-isoniazid) and isoniazid (Ingredient of rifampin-isoniazid)
(Major Drug-Drug Interaction)
MONITOR CLOSELY: The risk of hepatotoxicity is greater when rifampin and isoniazid are given concomitantly than when either drug is given alone. Rifampin appears to alter the metabolism of isoniazid and increase the amount of toxic metabolites. Theoretically, a similar reaction may occur with rifabutin and isoniazid. Patients who are elderly, have hepatic impairment, are slow acetylators of isoniazid, drink alcohol daily, are female, or are taking other strong CYP450-inducing agents may be at greater risk of hepatotoxicity.
MANAGEMENT: Close monthly monitoring for clinical or laboratory evidence of altered hepatic function is recommended. Patients should be advised to promptly report early symptoms of hepatitis such as fatigue, weakness, malaise, anorexia, nausea, or vomiting. Discontinuation of either or both drugs may be necessary.
• Rifampin and Pyrazinamide
(Major Drug-Drug Interaction)
The exact mechanism of interaction is unknown, although both agents are individually hepatotoxic and may have additive effects on the liver during coadministration.
MANAGEMENT:
• The American Thoracic Society and the Centers for Disease Control and Prevention recommend that the two-month RIF-PZA regimen generally not be offered to patients with LTBI
• Other acceptable options include nine months of twice-weekly INH, or six months of either daily or twice- weekly INH. Twice-weekly therapy must be administered under direct observed therapy (DOT)
• If RIF-PZA is prescribed, the PZA dosage should be no more than 20 mg/kg/day (up to a maximum of 2 g/day) or 50 mg/kg twice weekly (up to a maximum of 4 g twice weekly), and no more than a two-week supply of the medications should be dispensed at any given time.
• Patients should be evaluated in person by a healthcare provider at 2, 4, and 6 weeks of treatment for adherence, tolerance and adverse effects, and at 8 weeks to document treatment completion. Patients should also be instructed to discontinue the drugs promptly and seek medical attention if signs and symptoms of hepatic injury develop, including fever, rash, anorexia, nausea, vomiting, fatigue, right upper quadrant pain, dark urine, and jaundice.
• Serum transaminases and bilirubin should be measured at baseline and at 2, 4, 6, and 8 weeks of treatment in patients taking RIF-PZA. Therapy should be withdrawn and not resumed if transaminase levels exceed five times the upper limit of normal or are anywhere above the normal range when accompanied by symptoms of hepatitis, or if serum bilirubin is greater than the normal range.
• ethambutol and INH (isoniazid)
(Moderate Drug-Drug Interaction)
The risk of peripheral neuropathy may be increased during concurrent use of two or more agents that are associated with this adverse effect. In some cases, the neuropathy may progress or become irreversible despite discontinuation of the medications.
MANAGEMENT:
Caution is advised during concomitant use of agents with neurotoxic effects. Patients should be monitored closely for symptoms of neuropathy such as burning, tingling, pain, or numbness in the hands and feet. Since the development of peripheral neuropathy may be dose-related for many drugs, the recommended dosages should generally not be exceeded.
Consideration should be given to dosage reductions or immediate discontinuation of these medications in patients who develop peripheral neuropathy to limit further damage.
If necessary, therapy should generally be reinstituted only after resolution of neuropathy symptoms or return of symptoms to baseline status. In some cases, reduced dosages may be required.
16. GOALS OF THERAPY:
The main goal of therapy is to give symptomatic treatment of T.B and then start the definitive therapy for longer periods after examining the X-rays and other lab test reports. Diabetes control is necessary in such patient also. Elimination of causative bacterium and prevention of resistance should also be achieved.
• Patient should be continued with the treatment of Diabetes and Asthma with good compliance.
• Continue with the Anti T.B therapy with repeat tests of Liver Enzymes weekly.
• If liver enzymes continued to rise more than 2 times of upper normal limits then stop Rifampin, INH, Pyrazinamide and continue with Ethambutol with Streptomycin 1g I.M/d along with fluroquinolone (ciprofloxacin 500mg b.d.)
• Repeat L.F.T’s weekly and when it become normal, start adding either Rifampin, INH, or Pyrazinamide one by one and note the degree of elevation of Liver Enzyme by each drug.
• Eliminate the drug rising Liver enzyme from therapy at that time until Liver enzymes are normalized.
17. COUNSELLING WITH PATIENT:
Patients who take their TB treatment in an irregular and unreliable way are at greatly increased risk of treatment failure, relapse and the development of drug-resistant TB strains.
There are variety of reasons why patients fail to take their medication. The symptoms of TB commonly resolve within a few weeks of starting TB treatment and many patients then lose motivation to continue taking their medication. Regular follow-up is important to check on compliance and to identify any problems patients are having with their medication. Patients need to be told of the importance of taking their tablets regularly, and the importance of completing treatment, because of the risk of relapse or drug-resistance developing otherwise.
One of the main complaints is the bulkiness of the tablets. The main offender is PZA (the tablets being the size of horse tablets). PZA syrup may be offered as a substitute, or if the size of the tablets is truly an issue and liquid preparations are not available, then PZA can be omitted altogether. If PZA is omitted, the patient should be warned that this results in a significant increase in the duration of treatment (details of regimens omitting PZA are given below).
The other complaint is that the medicines must be taken on an empty stomach to facilitate absorption. This can be difficult for patients to follow (for example, shift workers who take their meals at irregular times) and may mean the patient waking up an hour earlier than usual everyday just to take medication. The rules are actually less stringent than many physicians and pharmacists realize: the issue is that the absorption of RMP is reduced if taken with fat, but is unaffected by carbohydrate, protein, or antacids. So the patient can in fact have his or her medication with food as long as the meal does not contain fat or oils (e.g., a cup of black coffee or toast with jam and no butter). Taking the medicines with food also helps ease the nausea that many patients feel when taking the medicines on an empty stomach. The effect of food on the absorption of INH is not clear: two studies have shown reduced absorption with food but one study showed no difference. There is a small effect of food on the absorption of PZA and of EMB that is probably not clinically important.
It is possible to test urine for isoniazid and rifampicin levels in order to check for compliance. The interpretation of urine analysis is based on the fact that isoniazid has a longer half-life than rifampicin:
• urine positive for isoniazid and rifampicin patient probably fully compliant
• urine positive for isoniazid only patient has taken his medication in the last few days preceding the clinic appointment, but had not yet taken a dose that day.
• urine positive for rifampicin only patient has omitted to take his medication the preceding few days, but did take it just before coming to clinic.
• urine negative for both isoniazid and rifampicin patient has not taken either medicine for a number of days
18. COUNSELLING WITH PRESCRIBER
• Inform doctor about the potential side effects and Drug interactions during the coarse of therapy.
• Suggest any deletion or addition of drugs in the selected long term regimen to avoid any hazardous effects e.g. Pyridoxine 25-50 mg PO qd should be co administered to prevent peripheral neuropathy while using Isoniazide.
• Advice doctor to monitor patients with active chronic liver disease or severe renal dysfunction; periodic ophthalmologic examinations during isoniazid therapy are recommended even when visual symptoms do not occur
• Tell doctor about the causative drug which increases the Liver Enzymes and kindly suggest alternative drug or about elimination of that drug until the liver enzymes become normal.
• Suggest any dose adjustment according to lab data.
19. RECOMMENDATION:
• Strict diabetes control is recommended during therapy.
• Rationale of each drug is made for patient and for hospital staff for better compliance and results.
• Proper medication should be done at regular devised time period.
• As the patient is having liver damage so Liver Supportive agents are recommended in the drug therapy in order to prevent further damage to liver such as Pyridoxine 25-50 mg PO qd should be co administered to prevent peripheral neuropathy while using Isoniazid.
• Iron Polymaltose or Vit-B Complex supplements are recommended for any anemic condition.
• Juices and sweet things are strictly controlled in order to control Hyperglycemia.
• Regular Blood sugar monitoring should be advised to patient and a record should be kept.
• Protein diet should be added in regular meal in order to increase anabolism.
• Vitamin D supplementation appears to have a beneficial effect on the treatment of tuberculosis and appears to enhance immunity to tuberculosis, and vitamin D deficiency is a risk factor for tuberculosis.
20. MONITORING OF THERAPY:
• During therapy liver enzymes should be closely monitored.
• Strict diabetes control is recommended during therapy.
• As the patient is having liver damage so Liver Supportive agents are recommended in the drug therapy in order to prevent further damage to liver such as Pyridoxine 25-50 mg PO qd should be co administered to prevent peripheral neuropathy while using Isoniazid.
• DOTS stands for “Directly Observed Therapy, Short-course” and is a major plank in the WHO global TB eradication programme. The DOTS strategy focuses on five main points of action. These include
1. government commitment to control TB,
2. diagnosis based on sputum-smear microscopy tests done on patients who actively report TB symptoms,
3. direct observation short-course chemotherapy treatments,
4. a definite supply of drugs, and
5. standardized reporting and recording of cases and treatment outcomes
• The WHO advises that all TB patients should have at least the first two months of their therapy observed: this means an independent observer watching tuberculosis patients swallow their anti-TB therapy.
• The independent observer is often not a healthcare worker and may be a shopkeeper or a tribal elder or similar senior person within that society. Self-administered therapy (SAT), is another form of treatment for tuberculosis, however it does not have a reliable efficiency rate.
2. ABNORMAL LFT’S:
Patient is treated with Rifampin, INH, Pyrazinamide combination. These drugs are liver enzyme inducers, and Bilirubin level is also increased. Elevations in bilirubin must be expected with RMP treatment (RMP blocks bilirubin excretion) and usually resolve after 10 days (liver enzyme production increases to compensate). Isolated elevations in bilirubin can be safely ignored.
Elevations in liver transaminases (ALT and AST) are common in the first three weeks of treatment. If the patient is asymptomatic and the elevation is not excessive then no action need be taken; some experts suggest a cut-off of four times the upper limit of normal, but there is no evidence to support this particular number over and above any other number. Some experts consider that treatment should only be stopped if jaundice becomes clinically evident.
3. ADDITION IN THERAPY:
a. As the patient is having liver damage so Liver Supportive agents are recommended in the drug therapy in order to prevent further damage to liver such as Pyridoxine 25-50 mg PO qd should be co administered to prevent peripheral neuropathy while using Isoniazid.
b. Iron Polymaltose or Vit-B Complex supplements are recommended for any anemic condition.
c. Juices and sweet things are strictly controlled in order to control Hyperglycemia.
4. PATIENT ADVICE:
• Tyramine- and histamine-containing foods should be avoided in patients receiving rifampin, isoniazid and Pyrazinamide.
• rifampin, may produce a reddish coloration of the urine, sweat, sputum, and tears, and the patient should be forewarned of this.
• Compliance with the full course of therapy must be emphasized, and the importance of not missing any doses must be stressed.
5. DRUG INTERACTIONS:
• Rifampin (Ingredient of rifampin-isoniazid) and isoniazid (Ingredient of rifampin-isoniazid)
(Major Drug-Drug Interaction)
MONITOR CLOSELY: The risk of hepatotoxicity is greater when rifampin and isoniazid are given concomitantly than when either drug is given alone. Rifampin appears to alter the metabolism of isoniazid and increase the amount of toxic metabolites. Theoretically, a similar reaction may occur with rifabutin and isoniazid. Patients who are elderly, have hepatic impairment, are slow acetylators of isoniazid, drink alcohol daily, are female, or are taking other strong CYP450-inducing agents may be at greater risk of hepatotoxicity.
MANAGEMENT: Close monthly monitoring for clinical or laboratory evidence of altered hepatic function is recommended. Patients should be advised to promptly report early symptoms of hepatitis such as fatigue, weakness, malaise, anorexia, nausea, or vomiting. Discontinuation of either or both drugs may be necessary.
• Rifampin and Pyrazinamide
(Major Drug-Drug Interaction)
The exact mechanism of interaction is unknown, although both agents are individually hepatotoxic and may have additive effects on the liver during coadministration.
MANAGEMENT:
• The American Thoracic Society and the Centers for Disease Control and Prevention recommend that the two- month RIF-PZA regimen generally not be offered to patients with LTBI
• Other acceptable options include nine months of twice-weekly INH, or six months of either daily or twice- weekly INH. Twice-weekly therapy must be administered under direct observed therapy (DOT)
• If RIF-PZA is prescribed, the PZA dosage should be no more than 20 mg/kg/day (up to a maximum of 2 g/day) or 50 mg/kg twice weekly (up to a maximum of 4 g twice weekly), and no more than a two-week supply of the medications should be dispensed at any given time.
• Patients should be evaluated in person by a healthcare provider at 2, 4, and 6 weeks of treatment for adherence, tolerance and adverse effects, and at 8 weeks to document treatment completion. Patients should also be instructed to discontinue the drugs promptly and seek medical attention if signs and symptoms of hepatic injury develop, including fever, rash, anorexia, nausea, vomiting, fatigue, right upper quadrant pain, dark urine, and jaundice.
• Serum transaminases and bilirubin should be measured at baseline and at 2, 4, 6, and 8 weeks of treatment in patients taking RIF-PZA. Therapy should be withdrawn and not resumed if transaminase levels exceed five times the upper limit of normal or are anywhere above the normal range when accompanied by symptoms of hepatitis, or if serum bilirubin is greater than the normal range.
• ethambutol and INH (isoniazid)
(Moderate Drug-Drug Interaction)
The risk of peripheral neuropathy may be increased during concurrent use of two or more agents that are associated with this adverse effect. In some cases, the neuropathy may progress or become irreversible despite discontinuation of the medications.
MANAGEMENT:
Caution is advised during concomitant use of agents with neurotoxic effects. Patients should be monitored closely for symptoms of neuropathy such as burning, tingling, pain, or numbness in the hands and feet. Since the development of peripheral neuropathy may be dose-related for many drugs, the recommended dosages should generally not be exceeded. Consideration should be given to dosage reductions or immediate discontinuation of these medications in patients who develop peripheral neuropathy to limit further damage. If necessary, therapy should generally be reinstituted only after resolution of neuropathy symptoms or return of symptoms to baseline status. In some cases, reduced dosages may be required.