G-protein Coupled Receptors
G-protein coupled receptors (GPCRs) represent the largest family of membrane proteins in the human genome. It is one of the richest sources of targets for new drugs for the pharmaceutical industry. There are seven transmembrane proteins in GPCRs; hence another common word for GPCRs is 7TM receptors.
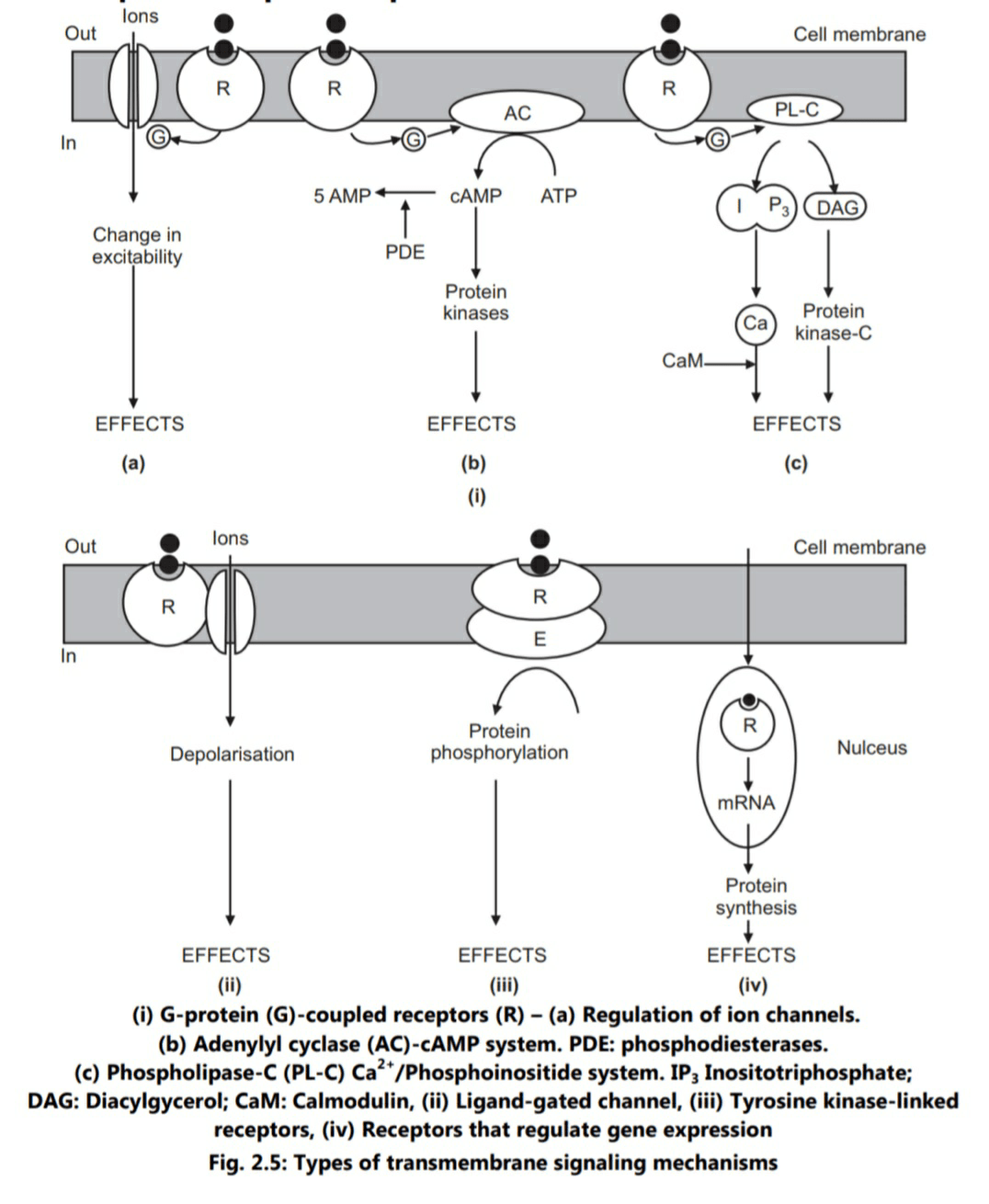
Many extracellular ligands act by increasing the concentration of intracellular second messengers, e.g. cAMP, Ca++ or phosphoionositides. Receptor occupancy activates G-protein which changes the activity of effector element (enzyme or ion channel). Finally there is change in the concentration of second messenger.
Various receptors like muscarinic cholinergic, adrenergic, histamine H2, serotonin (5HT), opiate and many peptide hormones (excluding insulin) belong to this family. Receptor occupancy by an appropriate ligand leads to activation of G-protein which in turn acts on target enzyme like adenyl cyclase/phospholipase C or an ion channel (Fig. 2.5).
G-proteins
Several varieties of G-proteins have been identified. The most common one are Gs and Gi. Gs and Gi stimulate or inhibit the enzyme adenyl cyclase respectively, thus producing opposite effects. Activation of adenyl cyclase increases concentration of cAMP while inhibition of the enzyme causes decrease in cAMP. β1-adrenergic amines, histamine H2 agonists, 5 HT1 agonists and polypeptide hormones stimulate Gs protein. On the contrary, β2-agonists, muscarinic M2 and δ-opioid receptor agonists act on Gi.
It is known that, a ligand-receptor interaction lasts only for milliseconds. Once a G-protein is activated, it remains so for about 10 seconds, during which the original signal is highly amplified. Thus, in G-protein-receptor coupled system, occupancy of only a fraction of receptor population is enough to produce maximal tissue response.
Physiological Roles
Following physiological roles have been identified for GPCRs:
• The visual sense: The opsins are used in photoisomerisation reaction to translate electro-magentic radiation into cellular signals; e.g. rhodopsin uses conversion of 11-cis-retinal to all-trans-retinal for this purpose.
• The gustatory sense (taste): GPCRs in taste cells mediate release of gustducin in response to bitter – and sweet-tasting substances.
• The sense of smell: Receptors of the olfactory epithelium bind odorants (olfactory receptants) and pheromones (vomeronasal receptors).
• Behavioural and mood regulation: Receptors in the mammalian brain bind several neurotransmitters like serotonin, dopamine, GABA and glutamate.
• Regulation of immune system activity and inflammation: Chemokine receptors bind ligands which mediate inter-cellular communication between cells of the immune system; receptors such as histamine receptors bind inflammatory mediators and engage target cell types in the inflammatory response. GPCRs are also involved in immune modulation and directly involved in suppression of TLR-induced immune response from T-cells.
• Autonomic nervous system transmission: Both sympathetic and parasympathetic nervous systems are regulated by GPCR pathways, responsible for control of many automatic functions in the body like blood pressure, heart rate and digestive processes.
• A cell-density sensing: A novel GPCR role exists in regulating cell-density sensing.
• Homeostasis modulation: Water balance is controlled through GPCR sensing.
• GPCR system is also involved in growth and metastasis of some types of tumours.
Classification of GPCRs
GPCRs can be grouped into six classes based on sequence homology and functional similarity:
• Class A (or 1) (Rhodposin-like)
• Class B (or 2) (Secretin family)
• Class C (or 3) (Metabotropic glutamate/pheromone)
• Class D (or 4) (Fungal mating pheromone receptors)
• Class E (or 5) (Cyclic AMP receptors)
• Class F (or 6) (Frizzled/Smoothened)
GPCR Structure
GPCRs are integral membrane proteins possessing seven membrane-spanning domains or transmembrane helices. The extra-cellular parts of the receptor can be glycosylated. Some seven-transmembrane helix proteins (channel rhodopsin) that resemble GPCRs may contain ion channels, with their protein. In 2007, the first structure of human GPCR was resolved. Human β2-adrenergic receptor GPCR structure is similar to bovine rhodopsin in terms of relative orientation of the seven-transmembrane helices. Structure-function relationship of GPCRs has been worked out. In addition, mechanism of action at molecular level is understood.
Second Messengers
There are four G-protein-coupled effector systems:
(i) Adenyl cyclase-cAMP system
The system has a wide variety of receptor population and produce diverse effects. The effects include ionotropic and chronotropic effects on heart, relaxation of smooth muscles, glycogenolysis, lipolysis. In addition, effects related to hormones like vasopressin, parathyroid hormone, ACTH, FSH, LH and TRH are mediated through the system. cAMP exerts most of these effects by stimulating cAMP – dependent protein kinases. The intracellular effects of cAMP are terminated by degradation of cAMP to 5-AMP by the enzyme phosphodiesterases (PDE). Methylxanthines like caffeine inhibit PDE and thus prolong the action of second messenger cAMP.
(ii) Phospholipase C-Ca++/phosphoionositide system
Some of the agonists which trigger this pathway are Acetyl choline (ACh M1), Catecholamines (a1), 5-HT2, TRH, Vasopressin (V1) and Angiotensin. This system is more complex than the cAMP pathway due to presence of two second messengers (inositotriphosphate, IP3 and diacylglycerol, DAG), and multiplicity of protein kinases.
(iii) cGMP system
cGMP, as a second messenger, has a signalling role only in few cell types. Receptorlinked- G-protein controls the enzyme guanylyl cyclase in a way similar to that of adenylyl cyclase. Activation of membrane-bound guanylyl cyclase leads to generation of cytosolic second messenger, cGMP which acts by stimulating a cGMP-dependent protein kinase. Increased cGMP concentration leads to relaxation of vascular smooth muscles. Ligands involved in this case are Acetyl choline, histamine, a peptide hormone-atrial natricuretic factor (ANF) and vascular endothelial nitric oxide. The system has also been detected in intestines.
(iv) Regulation of ion channels
G-protein coupled receptors can control some ion channels by mechanisms that do not seem to involve any second messenger. In this case, G-protein interacts directly with the channel; ACh M1 receptors enhance permeability of potassium ions in cardiac muscle, and opiate analgesics open potassium channels reducing neuronal excitability.