PHARMACEUTICAL CHEMISTRY
51. Which is NOT true for the substance with the following chemical structure?
A insoluble in water
B used as a topical, local anaesthetic
C a benzoic acid derivative
D insoluble in mineral acids
52. Sulfonamides are metabolised by humans principally by?
A acetylation
B deamination
C oxidation
D conjugation
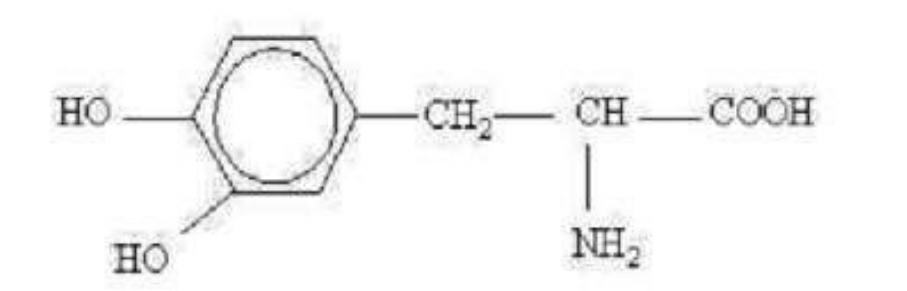
53. The compound with the following structure is (_)-3-(3,4-dihydroxyphenyl)-Lalanine. Select the most appropriate statement below.
A the compound is adrenaline
B it is a dextrorotatory compound
C it is a precursor of dopamine
D it is used to treat hypertension
54. in which drug is the pharmacological activity associated with specific optical isomer?
A adrenaline
B aspirin
C phenobarbitone
D acetylcholine
55. The functional group which contributes to the instability of aspirin is
A alcohol
B ketone
C ester
D heterocycle
56. The functional group which contributes to the instability of aspirin is
A alcohol
B ketone
C ester
D heterocycle
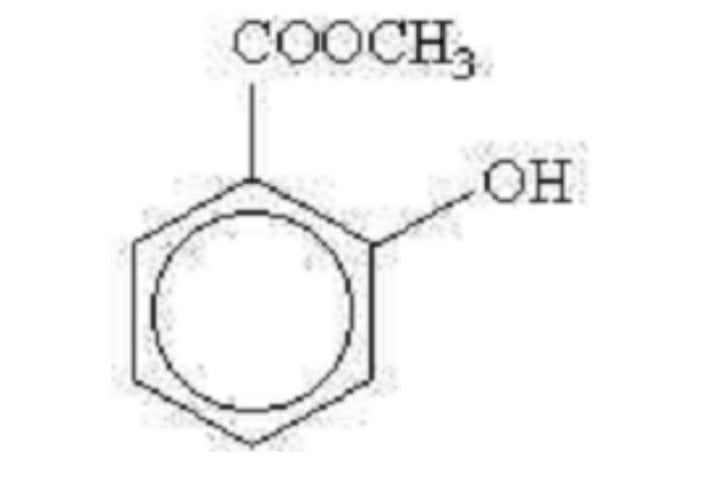
57. The chemical formula above represents
A aspirin
B methyl salicylate
C salicylic acid
D salicylamide
58. Which of the following compounds would have the highest boiling point?
(a) CH3CH2CH2CH3
(b) CH3NH2
(c) CH3OH
(d) CH2F2
59. Which of the following alkanes would have the highest boiling point?
Correct answer:- A
60.How the following compounds related?
(a) isoelectronic species
(b) isotopes
(c) isomers
(d) they all are totally different.
61. What is the total number of sigma bonds found in the following compound?
CH3 – CH = C= CH – C ≡ C – H
(a) 8
(b) 10
(c) 11
(d) 15
62. What is the total number of pi bonds found in the following compound?
H– C ≡ C – CH2 – NO2
(a) 1
(b) 2
(c) 3
(d) 4
63. 6% solution of urea is isotonic with
(A) 6% solution of Glucose
(B) 25% solution of Glucose
(C) 1 M solution of Glucose
(D) 0.05 M solution of Glucose
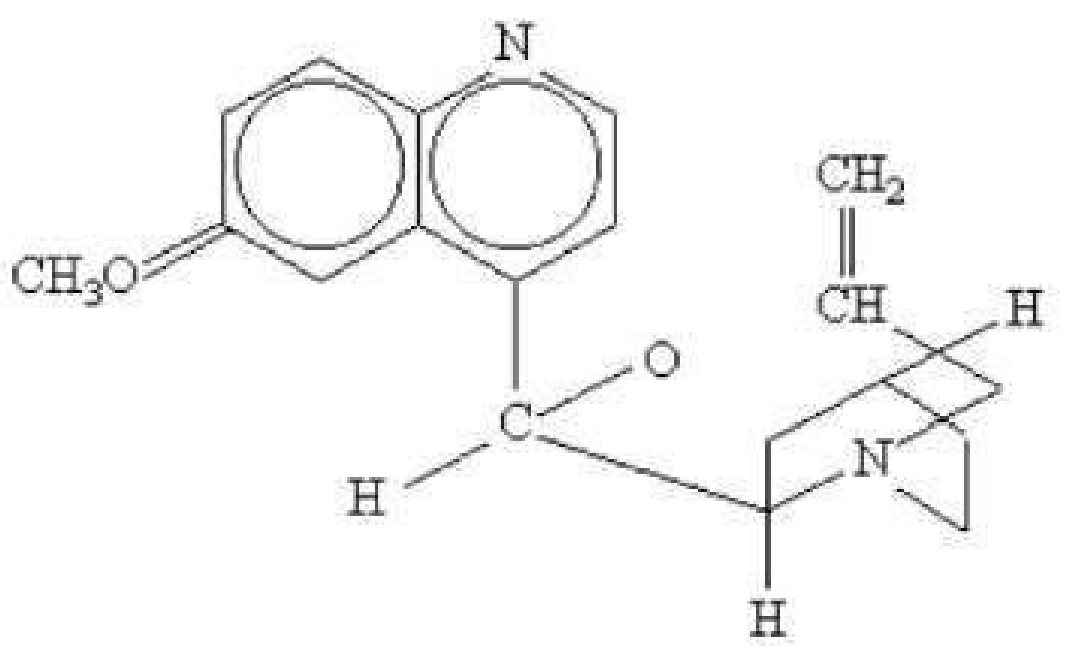
64. Which of the following therapeutic classifications does the chemical structure above belong to?
A tranquilizer
B anti-infective
C antihistamine
D analgesic
65. The structure pictured below is characteristic of
A cephalosporins
B thiazides
C thiobarbiturates
D penicillins
66. Identify the given structure.
A) Scopolamine
B) quinidine
C) morphine
D) none of this
67. Lactic acid is
A ethandioic acid
B dihydroxysuccinic acid
C 2-hydroxypropionic acid
D ethanoic acid
68. Aspirin is converted into salicylic acid in your body by which of the following reactions?
a) Hydrolysis
b) Oxidation
c) Reduction
d) Substitution
69. The structure of diamorphine differs from that of morphine by the addition of which functional group or groups?
a) One acetyl group
b) One methyl group
c) Two acetyl groups
d) Two methyl groups
70. What kind of antibacterial agent is erythromycin?
a) A macrolide
b) A penicillin
c) A cephalosporin
d) A tetracycline
71. You can usually tell if a drug is a natural product because
a) its structure is very simple
b) its structure contains lots of chiral centres and is very complex
c) you can’t tell just by looking at its structure
d) None of the above
72. Which of the following has the highest bond order?
(A) N2
(B) O2
(C) He2
(D) H2
73. Which of the following statements about alcohols is incorrect?
a) Tertiary alcohols have lower boiling points than primary alcohols with an equivalent molecular weight.
b) Alcohols undergo nucleophilic substitution.
c) Tertiary alcohols undergo dehydration more readily than primary alcohols.
d) Tertiary alcohols are metabolised in the body to ketones
74. Which of the following alcohols would be oxidised to propan-2-one?
a) ethanol
b) propan-2-ol
c) 2-methylpropan-2-ol
d) butan-1-ol
75. Which of the following statements about glycerol is incorrect?
a) It is metabolised to oxalic acid.
b) It is hygroscopic.
c) It can depress the freezing point of water.
d) It is a good solvent for polar covalent molecules.
76. Phenols can act as antioxidant because
a) they are acidic.
b) they undergo electrophilic substitution.
c) they are free radical scavengers.
d) they are easily oxidised.
77. Which of the following statements about ethers is incorrect?
a) Ethers are flammable.
b) Ethers form peroxides by free radical oxidation.
c) Ethers are widely used as extraction solvents.
d) An ether oxygen can only be found within an acyclic carbon chain.
78. Which of the following statements about ethylene oxide is incorrect?
a) It is a gas.
b) It is an acyclic ether.
c) It is highly toxic.
d) It is highly reactive.
79. Which of the following statements about the detoxification of haloalkanes is incorrect?
a) The normal detoxification process involves reaction with glutathione.
b) This detoxification process fails for aryl halides.
c) Haloalkanes react with glutathione in preference to other cell constituents.
d) Glutathione is a pentapeptide
80. Which of the following is a primary aromatic amine?
a) Aniline
b) Anisole
c) Anthracene
d) Adrenaline
81.Which of the following statements about amines is incorrect?
a) They react with acids to form salts.
b) Aliphatic amines are more basic than aromatic amines.
c) Primary amines are more basic than tertiary amines.
d) The biological oxidation of amines is usually carried out by monoamine oxidases.
82. Which of the following statements about quaternary ammonium compounds is incorrect?
a) They are neutral crystalline solids.
b) They are insoluble in water.
c) They are insoluble in lipids.
d) They are ionic molecules
83. What is a semi-synthetic drug?
a) A drug isolated from nature and used without any further modification
b) A drug made entirely in a lab from scratch
c) The structure of a drug half-way through its preparation
d) A drug which has been part-made by nature and part-made in a lab
84. Why may esomeprazole be considered a ‘me-too’ drug?
a) Because it is comparable in dose, dosage form, and intended use to an already known drug
b) Because it is manufactured by the same company as that of an already known drug
c) Because its structure is identical to an already known drug
d) Because it is a drug which works through the same mechanism as an already known drug
85. Which of the following is not an ore of magnesium?
(A) Carnallite
(B) Dolomite
(C) Calamine
(D) Sea water
86. Which of the following does not give benzoic acid on hydrolysis?
(A) phenyl cyanide
(B) benzoyl chloride
(C) benzyl chloride
(D) methyl benzoate
87. Which of the following is used to prepare Cl2 gas at room temperature from concentrated HCI?
(A) MnO2
(B) H2S
(C) KMnO4
(D) Cr2O3
88. 80g of oxygen contains as many atoms as in
(A) 80 g of hydrogen
(B) 1 g of hydrogen
(C) 10 g of hydrogen
(D) 5 g of hydrogen
89. A gas deviates from ideal behaviour at a high pressure because its molecules
(A) attract one another
(B) show the Tyndall effect
(C) have kinetic energy
(D) are bound by covalent bonds
90. A metal present in insulin is
(A) copper
(B) iron
(C) zinc
(D) aluminium
91. According to Bayer’s strain theory
which is highly stable?
(A) cyclohexane
(B) cycloheptane
(C) cyclopentane
(D) cyclobutane
92. Amines behave as
(A) Lewis acids
(B) Lewis base
(C) aprotic acid
(D) neutral compound
93. Which of the listed pharmaceuticals is not a hydrocarbon?
A. Benzinum
B. Paraffinum liquidum
C. Vaselinum album
D. Cetaceum
94. Which of the following compounds are starting materials in the SOLVAY process of soda production?
A. sodium sulfate
B. ammonia
C. sodium chloride
D. carbon monoxide
95. Among the following, the compound that contains ionic, covalent and coordinate linkage is
(A) NaCl
(B) CaO
(C) NH3
(D) NH4Cl
96. Among these, which is least acidic?
(A) phenol
(B) O-cresol
(C) p-nitrophenol
(D) p-chlorophenol
97. An alkyl halide reacts with alcoholic ammonia in a sealed tube, the product formed will be
(A) a primary amine
(B) a secondary amine-
(C) a tertiary amine
(D) a mixture of all the three
98. An element with atomic number 21 is
(A) halogen
(B) representative element
(C) transition element
(D) alkali metal
99.Which of the following solutions is buffer?
a) Acetic acid and sodium acetate
b) Acetic acid and sodium sulfate
c) Hydrochloric acid and sodium sulfate
d) Hydrochloric acid and sodium chloride
100. The sp3d2 hybridization of central atom of a molecule would lead to
(A) square planar geometry
(B) Tetrahedral geometry
(C) Trigonal bipyramidal geometry
(D) Octahedral geometry