PACKAGING:
It is the art, science and technology to enclose and protecting the material. It is done so that the product can retain its therapeutic effectiveness from the time of manufacturing to the time of consuming.PHARMACEUTICAL PACKAGING:
The combination of components required to contain, preserve, protect and deliver a safe, efficacious product at any time point before expiration dating is called as pharmaceutical packaging.Types of packaging:
Primary packaging:
The packaging which is in direct contact with the formulation held in it is called as primary packaging. For example, a glass bottle is in direct contact with the syrup.
Secondary packaging:
The packaging which is not in direct contact with formulation or which is used to provide protection to the primary packaging and formulation is called as secondary packaging. Example: Cartoon packaging.
Tertiary packaging:
It is an another type of packaging which is generally used when a large number of containers having product have to be transport from one place to another place. Example: 100 containers of syrups are packed in one cartoon and then transport to another place.
Ideal properties for a packaging material:
• It should be stable.
• It should be compatible with contents.
• It should be able to protect the formulation.
• It should be non-toxic.
• It should not reactive with the formulation.
• It should be FDA approved.
• It should be impermeable to moisture, oxygen and other gases.
• It should provide protection against light.
• It should be cost effective.
• It should be resistant to corrosive liquids and other solvents.
• It should have proper strength.
Functions of packaging:
Protection: It should provide protection against all external environmental conditions which may affect its quality or potency like moisture. Light, oxygen etc. Product identification: It should help in identification of product contain in it.
Facilitating the use of product: It should be easy to open, handle and use by the consumer.
Product promotion: It is used for promotional and attracting the attention of the people while purchasing.
MATERIAL USED FOR PACKAGING
Glass: It is commonly used material for packaging of pharmaceuticals. It is generally composed of:
• SiO2(sand), Na2CO3(Soda ash) and CaCO3(Lime stone) and cullet (Fusing agent in glass)
The high melting point of glass is due to presence of silica and it can be modified by addition of various oxides. Decrease in amount of sodium in glass, increases the chemical resistant power of glass but without sodium or potassium glass is difficult or expensive to manufacture. Boron oxide is added in glass to achieve melting point of glass. Lead is added in glass to increase the clarity of the glass but it may also produce soft glass. Aluminium oxide is added in glass to increase the hardness and durability of the glass.
Manufacturing of glass: Following four processes are used for manufacturing of glass:
1. Blowing: It uses compressed gas to convert the melted glass into cavity of a mold.
2. Drawing: In this melted glass is pulled through dies or rollers to give it shape.
3. Pressing: In this, mechanical force or pressure is applied to melted glass in the cavity of a mold.
4. Casting: In this, gravity or centrifugal force is used to convert the melted glass into the cavity of mold.
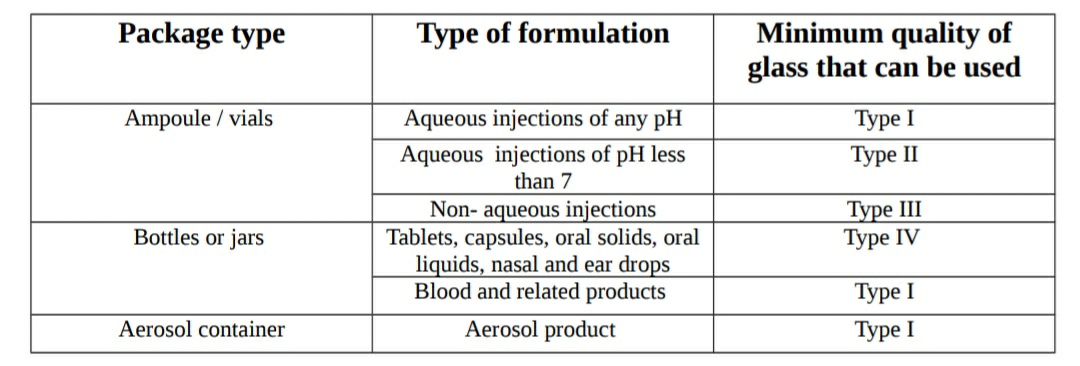
Types of glass: Glass is of four types:
1. Type I glass (Borosilicate glass or highly resistant glass)
2. Type II glass (Treated soda lime glass)3. Type III glass (Soda lime glass)
4. Type IV (General purpose glass or non-parenteral glass)
Type I or borosilicate glass:
It is highly resistant or chemically inert glass. It is generally composed of:
Silicon dioxide: 70-80%
Boron oxide: 4-8%
Sodium oxide: 2-8%
Aluminium oxide: 2-8%
Generally addition of 6% boron in borosilicate glass decreases the leaching of alkali in formulation.
Uses: It is used for storage of strong acids or strong alkalis or all type of solvents. It is generally used for packaging of parenteral products.
Type II or treated soda lime glass:
It is a modified glass III (soda lime glass) having high hydrolytic resistance due to treatment of inner surface of soda lime glass with sulfur. It is generally done to remove leachable oxides and thus prevent the problem of blooming or weathering of the glass. Type II glass have lower melting point than type I glass and can be easily manufactured.
Use: It is used for packing of neutral and acidic parenteral products.
Type III or soda lime glass:
It is an untreated soda lime glass having average chemical resistance. It is generally composed of around 75% SiO2, 15% NaO, 10% CaO and small amount of aluminium oxide, magnesium oxide and potassium oxide.
Use: It is generally used for packing the dry powders for parenteral use only when satisfactory stability data is available.
Type IV or non-parenteral glass:
This type of glass has very low hydrolytic resistance. It is not suitable for packaging of parenetral products and hence it is used for packaging of general products like tablets, capsules, syrups etc.
Colored glass or amber colored glass: This type of glass is generally used for packaging of photosensitive products because these types of products degrade in presence of ultraviolet light present in sunlight. These type of glass should provide protection at light wavelength of 290-450nm. Iron oxide used to manufacture amber color glass leach out in formulation if it contains iron catalyzed ingredients.
Advantages of glass:
• It is quite strong and rigid.
• It is transparent enable visual inspection of product in it.
• It can be molded into various shapes and sizes.
• It is impermeable and chemically inert.
• It does not deteriorate with age.
• It has proper closure system.
• Photosensitive drugs can be avoided from degradation by amber color glass.
• It can be easily cleaned without damaging the surfaces.
• It is comparatively cheaper.
Disadvantages:
• It is fragile.
• It may crack on exposure to sudden change in temperatures.
• It is heavier than plastic.
• Sunlight can degrade the products in transparent glass.
• Flaking (Leaching alkali from inner surface of glass) is possible.
• Blooming of weathering occur in soda lime glass.
Plastic:
These are synthetic polymers of high molecular weight. These are more sensitive to heat and may soften or melt at or below 100°c. Plastic used as packaging material is of two types:
Thermoplastic: This plastic, soften or melt in a viscous fluid on heating and hardens again on cooling. Example: Polyethylene, Polystyrene, Polyvinyl chloride, Nylon, Polycarbonate etc.
Thermosetting: This plastic become flexible or soft on heating but does not become liquid. Example: Phenol-formaldehyde, Urea-formaldehyde etc.
Additives used in plastic:
These are other excipients which are added in plastic to make it desirable for packaging.
Stabilizers
• Antioxidants
• Pigments
• Fillers
• Plasticizers
• Other agents like curing agent, cross linking agent, accelerator etc.
Examples of some plastic used as packaging material:
Polyethylene:
High density polyethylene (HDPE) is generally used as packaging material for pharmaceutical products. It is good barrier against moisture but poor barrier against oxygen and various gases hence antioxidant is added. Antioxidant used is butylated hydroxyl toluene and antistatic agent is polyethylene glycol in 0.1-0.2% concentration. It lacks clarity and causes permeation of essential odors, flavors etc from the product. The density of polyethylene is 0.91-0.96 and it determines the stiffness, water vapor permeability, stress cracking and clarity of polyethylene. Increase in density of plastic stiffness, water vapor permeability and stress cracking are improved.
Polypropylene:
Polypropylene is more popular than polyethylene because it does not undergo cracking on applying stress. It shows resistance to most of the chemicals except halogenated solvents. It has high melting point which makes it ideal for packaging the sterilizable products. It has greatest disadvantage that it become brittle at low temperature or become fragile at 0°F. Hence it is generally used in combination with polyethyle
Polyvinyl chloride (PVC):
The containers made up of PVC are clear, rigid, impermeable to oxygen and has good stiffness. It can be softened by addition of plasticizers. PVC is inexpensive, tough, clear and can be easily prepared. It starts to degrade above 280°F hence it should be overheated. It becomes yellow on exposure to ultraviolet light or heat hence stabilizer is added. Stabilizer is Diocetyl-tin merceptoacetate. It can cause liver cancer due to the presence of vinyl chloride in its composition.
Polystyrene:
It is generally used for packaging of solid dosage forms and it is not useful for liquid dosage form. It has high water vapor permeability as well as oxygen permeation. It has low melting point of 190°F hence not suitable for sterilizable items. It is used in combination with varying concentration of rubber and acrylic compounds.
Nylon (Polyamide):
It can be autoclaved, strong and quite difficult to destroy by mechanical method. It is resistant to variety of organic and inorganic chemicals. It is also highly impermeable to oxygen. Nylon film can be laminated to polyethylene and other type of plastic.
Polycarbonate: It is a clear transparent plastic which can be repeatedly sterilized. It is rigid like glass hence considered as substitute for glass ampoule and vials. It is resistant to dilute acids, oxidizing and reducing agents, salts, oils and aliphatic hydrocarbons. Polycarbonate resins are expensive and hence used in special containers. The strength of polycarbonate is 5 times greater than other plastic containers.
Advantages:
• It is economical.
• It is light in weight.
• It is durable.
• It is pleasant to touch.
• It is odourless and inert to various chemicals.
• It is not fragile.
• It is able to retain its shape during use.
Disadvantages:
• It causes leaching (Release to constituent from the plastic into the formulation).
• It causes permeation (The transmission of gases, vapors, moisture from surrounding to the formulation in container)
• It causes sorption (Absorption of constituents of formulation by the container)
• It causes chemical reaction with ingredients of formulation.
• It causes modification (Physical and chemical changes in the packaging material by the formulation)
Rubber:
Rubber of varying composition is used in pharmaceuticals and biological products as closure, cap liner or part of dropper. Natural rubber consists of long chain polymers of isoprene units linked together at cis-position. The natural rubber is called as latex and obtained from the tree Hevea braziliensis. The rubber is generally used for making the closure for vials having multidose injections and solutions. The major problem with rubber is sorption of ingredients of the formulation.
Additives used in rubber:
Vulcanizing agent: It is added in rubber to increase the hardness of the rubber. Example: Sulphur
Accelerator: It is added to decrease the time of vulcanization of to increase the vulcanization. Example: 2-mercapto benzthiazol (MBT), Zinc dimethyl dithiocarbamate.
Activator: It is added to increase the activity of accelerators. Example: Stearic acid, zinc oxide
Fillers: Two types of fillers are used in rubber:
Reinforcing fillers: These are added to modify the physical properties of the rubber. Example: Carbon black, magnesium carbonate, calcium carbonate
Extending filler: These are added as diluents to decrease the cost of rubber. Example: Talc, Asbestos
Softeners: These are added to make manufacturing easier. Example: Pine oil, Mineral oil
Antioxidant: These are added to prevent the oxidation of rubber. Example: P-hydroxy diphenyl.
Pigments: These are added to provide desired color to the rubber. Example: Oxides of iron, coal tar dyes.
Lubricants: These are added in rubber to make easier the removal of rubber from the mold. Example: Zinc stearate, Stearic acid.
Metal: Metal containers are used when strength or malleability is required like in collapsible tube. Generally tin, aluminium, steel etc are used as metal for packaging of pharmaceuticals.
Tin: Tin is mellable, ductile, high crystalline and non-reactive metal. It is generally used for food or pharmaceutical products. Tin is used in production of aerosol container by electroplating it on steel sheet to increase corrosion resistance. It provides good appearance and compatibility with a wide range of products. But it has a disadvantage that tin corroded by chlorides or acids hence vinyl and cellulose liquids are used to increase their utility.
Aluminium: It is light in weight and hence shipment cost of the product is reduced. Coated and uncoated aluminium tubes are used. Aluminium reacts with fatty alcohol emulsions and form white layer on it. These are not used for storage of mercury containing compounds. Uncoated aluminium tubes caused harmful effect when used for preparations having pH range outside the 6.5-8.0.
Lead: It is commonly used for storage of non-food products like adhesives, inks, paints etc. due to lower cost among all metals. It should never be taken alone internally because it may cause lead- poisoning. Lead tubes are used for products like fluoride toothpaste by internal lining.
Factors affecting the choice of container: Following factors should be considered during selection of container or packaging system for pharmaceutical formulations:
Protection: The container closure system used for packaging should be able to protect the formulation from external hazards like oxygen, moisture, light radiations etc. Example: For photosensitive drugs, amber colored container should be used otherwise it may cause degradation of drug product.
Type of product: Type of product or formulation should be considered during selection of container closure system. Example: Aqueous injections cannot be filled in type III glass because it may cause deterioration of it. The product to be sterilized cannot be filled into the polyethylene due to low melting point of the polyethylene.
Compatibility: The container closure system used for pharmaceutical formulation should be compatible with ingredients of the formulation otherwise it may cause undesirable changes in the formulation causing degradation of it. Example: The iron catalyzed chemicals cannot be filled into the amber colored container because it causes leaching of iron oxide from the container.
Safety: The material used for manufacturing of container closure system should be non-toxic and should not give any odor or color to the formulation contained in it. The material should be eco-friendly and economical.
Performance: The container closure system should be adaptable with common high speed packaging machines and should be provide temper-resistant packaging when needed.
Route of administration: Route of administration also affects the choice of container. If the formulation has to be administered by parenteral route then Type –IV glass cannot be used. Physical factors: The container should have sufficient strength to withstand the pressure, temperature and other stresses during processing.
Sterilization: If the formulation has to be sterilized repeatedly then it cannot be filled into the polyethylene, polystyrene container, then only polycarbonate containers can be used in plastic.
Cleaning: It should be considered that container used for product should be easily cleanable to prevent the contamination of pharmaceutical products.
Transparency: The container used for storage of pharmaceutical products should be transparent for visual inspection of the content during the process or after finishing the process.
Cost: The container used for storage of products should be cost effective or economical to avoid the ultimate final cost of the product.
