Propranolol
Structure –
Propranolol is a medication that belongs to the class of drugs known as beta-blockers. It is used to treat high blood pressure, irregular heartbeats, and other cardiovascular conditions.
The chemical structure of propranolol can be represented using the following molecular formula and structural diagram:
Molecular formula: C16H21NO2
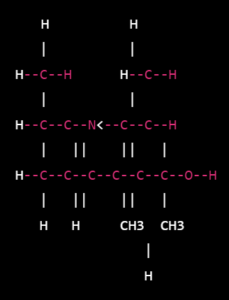
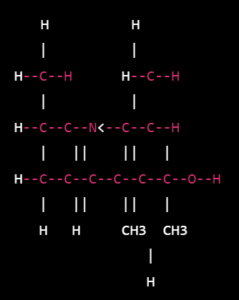
Structural diagram:
- In this diagram, the black lines represent covalent bonds between atoms, and the small letters next to the atoms indicate their chemical symbol. The larger letters outside the diagram represent functional groups or substituents.
- Propranolol contains a central propanolamine moiety (highlighted in blue), which is linked to a naphthalene ring (highlighted in green) through a bridging carbon atom. The naphthalene ring contains two benzene rings fused together. The molecule also contains a secondary amine functional group (-NH-) and a hydroxyl group (-OH) attached to the naphthalene ring.
- The stereochemistry of propranolol is important for its activity, as it is a racemic mixture of two enantiomers. The S(-)-enantiomer is responsible for most of its beta-blocking activity.
Synthesis –
Propranolol can be synthesized through a multistep process, starting from commercially available starting materials. The following is a simplified overview of the synthesis:
Step 1: Synthesis of 1-naphthol 1-naphthol can be synthesized from naphthalene through a process known as nitration, followed by reduction. In this process, naphthalene is first nitrated using a mixture of nitric and sulfuric acids, which adds a nitro group (-NO2) to one of the rings to form 1-nitronaphthalene. The nitro group is then reduced using a metal catalyst such as iron or zinc and hydrochloric acid, to yield 1-naphthol.
Step 2: Protection of 1-naphthol The hydroxyl group of 1-naphthol is protected using a protecting group such as benzyl bromide and sodium hydride, to prevent unwanted reactions from occurring at this site during subsequent steps.
Step 3: Synthesis of 3-(isopropylamino)-1-chloropropane 3-(isopropylamino)-1-chloropropane can be synthesized from isopropanol and epichlorohydrin through a process known as a halohydrin reaction. In this process, isopropanol is treated with sodium hydroxide to form the corresponding alkoxide, which is then reacted with epichlorohydrin to yield 3-(isopropyl)-1-chloropropanol. This intermediate is then reacted with diisopropylamine and triethylamine to form 3-(isopropylamino)-1-chloropropane.
Step 4: Coupling of protected 1-naphthol and 3-(isopropylamino)-1-chloropropane The protected 1-naphthol and 3-(isopropylamino)-1-chloropropane are coupled using a base such as sodium hydride, to form the desired product 1-(isopropylamino)-3-(1-naphthyloxy)-2-propanol. The benzyl protecting group is then removed using hydrogenation with palladium on carbon and hydrogen gas.
Step 5: Resolution of enantiomers The resulting product is a racemic mixture of two enantiomers, which can be separated using chiral chromatography or by enzymatic resolution. The S(-)-enantiomer is the active form of propranolol, and can be isolated and purified for use as a drug.
SAR –
The structure-activity relationship (SAR) of Propranolol is based on the modification of the chemical structure of the compound to enhance its pharmacological properties. The SAR of Propranolol can be summarized as follows:
- Aromatic ring: The aromatic ring in the structure of Propranolol is important for its pharmacological activity. Substitution at the 1-position of the ring with a bulky group such as isopropyl or t-butyl enhances the beta-blocking activity of the compound.
- Hydroxyl group: The hydroxyl group at the 4-position of the aromatic ring is important for the beta-blocking activity of Propranolol. Substitution or removal of this group decreases the beta-blocking activity of the compound.
- Amino group: The amino group at the 2-position of the structure of Propranolol is important for its pharmacological activity. Substitution of this group with larger groups such as ethyl or propyl decreases the beta-blocking activity of the compound.
- Propranolol enantiomers: Propranolol has two enantiomers, S(-) and R(+), which have different pharmacological properties. The S(-) enantiomer is the active form of Propranolol and has higher beta-blocking activity compared to the R(+) enantiomer.
- Lipophilicity: The lipophilicity of Propranolol is an important factor that affects its pharmacokinetics and pharmacodynamics. Increasing the lipophilicity of the compound enhances its absorption and distribution, but may also increase the risk of adverse effects and toxicity.
In summary, the SAR of Propranolol highlights the importance of the aromatic ring, hydroxyl group, amino group, enantiomers, and lipophilicity in determining the pharmacological properties of the compound.
Mechanism –
it is a non-selective beta-blocker that works by blocking beta-adrenergic receptors in the body. It has both negative chronotropic (reduces heart rate), negative inotropic (reduces force of contraction), and negative dromotropic (slows conduction of electrical impulses through the heart) effects on the heart.
The mechanism of action of Propranolol can be summarized as follows:
- Beta-adrenergic receptor blockade: Propranolol blocks beta-adrenergic receptors, which are located in various tissues in the body, including the heart, lungs, and blood vessels. By blocking these receptors, Propranolol reduces the effects of the sympathetic nervous system, which is responsible for the “fight or flight” response. This leads to a decrease in heart rate, cardiac output, and blood pressure.
- Antiarrhythmic effects: Propranolol has antiarrhythmic effects due to its ability to block beta-adrenergic receptors in the heart. By reducing the effects of sympathetic stimulation on the heart, Propranolol can help to control or prevent arrhythmias, particularly those related to excessive sympathetic activity.
- Decreased oxygen demand: Propranolol can reduce oxygen demand by decreasing heart rate and contractility, which in turn reduces myocardial oxygen consumption. This is particularly useful in the treatment of angina pectoris, where the reduction in oxygen demand can help to relieve symptoms.
- Central nervous system effects: Propranolol can also have effects on the central nervous system, particularly in the treatment of anxiety and tremors. By blocking beta-adrenergic receptors in the brain, Propranolol can reduce the effects of sympathetic activity and help to control symptoms.
In summary, it works by blocking beta-adrenergic receptors, leading to a decrease in sympathetic activity and a reduction in heart rate, cardiac output, and blood pressure. This mechanism of action is responsible for the therapeutic effects of Propranolol in the treatment of various cardiovascular and non-cardiovascular conditions.
Uses –
Propranolol is a non-selective beta-blocker that is used for the treatment of various medical conditions. Some of the main uses of it are:
- Hypertension: it is used to lower blood pressure in people with hypertension. It works by blocking the effects of the sympathetic nervous system on the heart and blood vessels, leading to a reduction in blood pressure.
- Angina pectoris: it is used to treat angina pectoris, which is chest pain that occurs due to inadequate blood supply to the heart. Propranolol reduces the heart rate and contractility, which decreases the oxygen demand of the heart and relieves angina symptoms.
- Arrhythmias: it is used to treat various arrhythmias, including supraventricular tachycardia, atrial fibrillation, and ventricular arrhythmias. It works by reducing the effects of sympathetic stimulation on the heart, which can help to control or prevent arrhythmias.
- Migraine prophylaxis: it is used for the prophylaxis of migraines, a common type of headache that is characterized by severe pain, nausea, and sensitivity to light and sound. Propranolol can reduce the frequency and severity of migraines by reducing the activity of the sympathetic nervous system.
- Essential tremor: it is used to treat essential tremor, a neurological disorder that causes involuntary shaking of the hands, head, and voice. Propranolol can reduce the severity of tremors by blocking the effects of the sympathetic nervous system.
- Anxiety: it is sometimes used for the treatment of anxiety, particularly in situations where physical symptoms such as tremors or sweating are present. It works by blocking the effects of the sympathetic nervous system, which can reduce the physical symptoms of anxiety.
In summary, Propranolol is a versatile medication that is used for the treatment of hypertension, angina pectoris, arrhythmias, migraine prophylaxis, essential tremor, and anxiety. Its non-selective beta-blocking action makes it effective in controlling the effects of the sympathetic nervous system on various organs in the body.