Tolazoline
Structure-
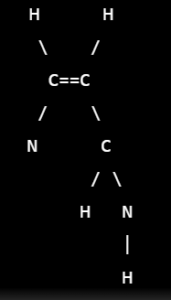
it is a drug that belongs to the class of alpha-adrenergic blocking agents. Its molecular formula is C10H12N2 and its structural formula is:
it has a bicyclic structure with two nitrogen atoms in the ring system. It has a piperidine ring fused to a pyrimidine ring. The pyrimidine ring contains a double bond between two carbon atoms, while the piperidine ring has a secondary amine functional group. The molecule also has two methyl groups attached to the pyrimidine ring.
Synthesis –
There are several methods for the synthesis of tolazoline, but one of the most common methods is as follows:
- The reaction between 2-methylpyrimidine and 2-aminopiperidine in the presence of a catalyst such as sodium methoxide to form 2-methyl-5-(2-piperidyl)pyrimidine.
- The resulting product is then treated with benzaldehyde and sodium borohydride to produce tolazoline.
The overall reaction can be summarized as follows:
2-methylpyrimidine + 2-aminopiperidine → 2-methyl-5-(2-piperidyl)pyrimidine
2-methyl-5-(2-piperidyl)pyrimidine + benzaldehyde + NaBH4 → tolazoline
Note that this is just one example of a synthesis route for tolazoline and there may be other variations or modifications to this method depending on the specific conditions and starting materials used.
or
The synthesis of tolazoline can be represented as follows:
Step 1: Synthesis of 2-methyl-5-(2-piperidyl)pyrimidine
2-methylpyrimidine and 2-aminopiperidine are reacted in the presence of a catalyst, such as sodium methoxide, to form 2-methyl-5-(2-piperidyl)pyrimidine:
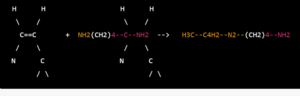
H2N(CH2)4NH2 + CH3C4H3N2 → CH3C4H2N2(CH2)4NH2
Step 2: Synthesis of tolazoline
The resulting 2-methyl-5-(2-piperidyl)pyrimidine is then reacted with benzaldehyde and sodium borohydride to form tz:
CH3C4H2N2(CH2)4NH2 + C6H5CHO + NaBH4 → C10H12N2 + NaBO2 + 2H2O
H3C–C4H2–N2–(CH2)4–NH2 + C6H5CHO + NaBH4 –> H3C–C4H2–N2–(CH2)4–N–C6H5 + NaBO2 + 2H2O
Overall, the synthesis of tolazoline involves the condensation of 2-methylpyrimidine and 2-aminopiperidine to form 2-methyl-5-(2-piperidyl)pyrimidine, which is then reacted with benzaldehyde and sodium borohydride to yield tolazoline.
SAR –
The Structure-Activity Relationship (SAR) of tolazoline refers to how changes in the chemical structure of the molecule can affect its biological activity. Here are some SAR considerations for tolazoline:
- Piperidine ring: The presence of the piperidine ring in tolazoline is important for its activity as an alpha-adrenergic blocking agent. Substituents on the piperidine ring, such as alkyl or aryl groups, can affect the potency and selectivity of the drug.
- Pyrimidine ring: The pyrimidine ring in tolazoline is also important for its activity. The double bond between two carbon atoms in the pyrimidine ring is necessary for the drug’s activity as an alpha-adrenergic blocker.
- Amino group: The secondary amino group on the piperidine ring is also important for the activity of tolazoline. Modifications to this group can affect the drug’s potency and selectivity.
- Methyl groups: The two methyl groups attached to the pyrimidine ring do not appear to play a significant role in the drug’s activity as an alpha-adrenergic blocking agent.
Overall, the SAR of tolazoline suggests that modifications to the piperidine ring and the amino group on that ring can affect the drug’s potency and selectivity as an alpha-adrenergic blocking agent.
Mechanism –
IT is an alpha-adrenergic blocking agent that acts by inhibiting the action of norepinephrine and other catecholamines on alpha-adrenergic receptors. The exact mechanism of action of tolazoline is not fully understood, but it is thought to involve several steps:
- IT binds to the alpha-adrenergic receptors on smooth muscle cells.
- This binding prevents the binding of norepinephrine or other catecholamines to the receptor.
- As a result, the signal for vasoconstriction or other alpha-adrenergic effects is blocked, leading to vasodilation and a decrease in blood pressure.
- TZmay also have additional mechanisms of action, such as blocking calcium channels and reducing intracellular calcium levels, which contribute to its vasodilatory effects.
Overall, tolazoline’s mechanism of action involves blocking the activity of alpha-adrenergic receptors, which leads to vasodilation and a decrease in blood pressure.
Uses –
it has several medical uses, including:
- Treatment of persistent pulmonary hypertension of the newborn (PPHN): it is used in the treatment of PPHN, a condition in which the newborn’s pulmonary arteries are narrowed and obstructed, leading to decreased oxygenation of the blood. Tolazoline acts as a vasodilator, helping to increase blood flow to the lungs and improve oxygenation.
- Reversal of local anesthesia: it can be used to reverse the effects of local anesthesia in the mouth or skin. It acts by constricting the blood vessels in the affected area, which helps to reduce the spread of the anesthetic and hasten its metabolism and elimination.
- Diagnosis of pheochromocytoma: it is used in the diagnosis of pheochromocytoma, a rare tumor of the adrenal gland that secretes catecholamines. Tolazoline is given intravenously, and if the patient has pheochromocytoma, it can cause a sudden release of catecholamines, leading to a characteristic rise in blood pressure.
- Investigational use in COVID-19: it is currently being investigated as a potential treatment for COVID-19, as it has been shown to have antiviral and immunomodulatory effects in laboratory studies. However, further research is needed to determine its efficacy and safety in humans.
Overall, it is used for its vasodilatory effects, as well as for its ability to reverse local anesthesia and assist in the diagnosis of certain medical conditions.