VALIDATION MASTER PLAN
• Validation master plan is also known as VMP
• It is key document in GMP (Good Manufacturing Practice).
• It describes the key elements of the validation programme, organizational
structure of validation, schedules and responsibilities.
• It should describe: “Why, What, Where, by whom, how and when?”
Scope
• Reduce rework
• Satisfactory inspections
• Helps in new drug approval
Contents of VMP
• Introduction
• Methodology
• Qualification
• Personnel
• Schedule
• Preventative maintenance
• Change control
• Procedure
• Documentation
• Appendices
INTRODUCTION:
It include following details:
• A description of facility, premises, Equipment & its purpose
• Scope of validation
• Policies on regulatory bodies like GMP, cGMP, WHO.
Methodology:
• Predetermined requirement to identifying the standards.
• Development of the acceptance criteria that are used to judge the validation
• It is also involve planning and execution of documents such as protocols, records, reports, or other.
• The std will involve three elements
1. Regulatory and guidance documents
2. National standards
3. Company standards
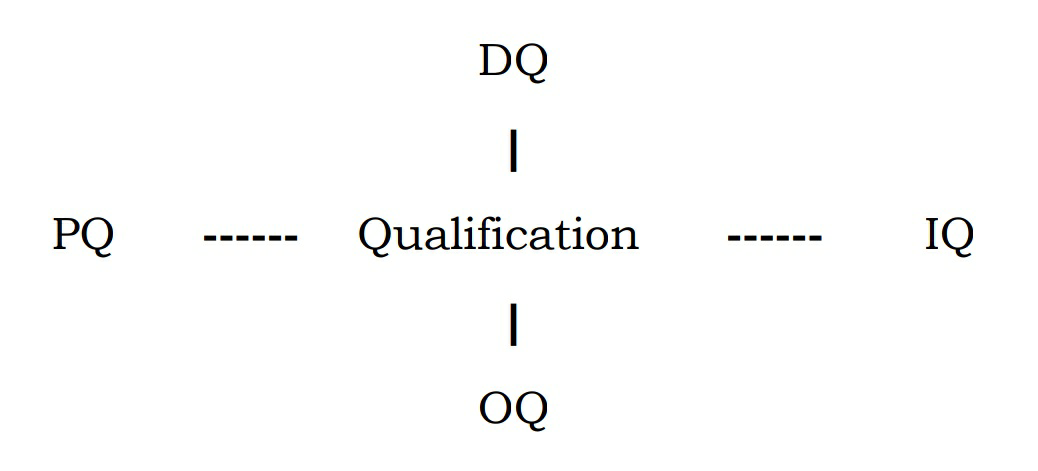
Qualification:
• Includes all the aspects of Design, procurement, installation, and
commissioning process
• It is important to ensure that the organization is consistent and cover all the aspect of validation process for specific project.
• The validation structure and organization is clear to any inspection authority.
• Design qualification providing documented evidence that the design of facility and equipment meet the requirements of the user specification & GMP.
Personnel:
• Each person engaged in each person responsible for supervising the manufacture Processing, packaging, or holding of a drug product shall have the education, training, and experience or a combination there of enable that person to perform the assigned functions”
✓ Principles for personnel requirements.
✓ Experience of personnel in house training reports, etc.
Schedules:
• Essential
• Prepared at early stage
• A good plan contains all necessary features which are to be considered during execution of plan and determines the control of the project.
• It ensures that the personnel involved in the VMP are not only aware of the
engineering targets, but also the validation targets.
Preventive Maintenance:
• This is the responsibility of site maintenance and operation dept.
• The activity should be performed during the design phase, and documentation required should be, included in requisition
Change control:
• This section of VMP should lay down requirements for a set of procedures for
change control that cover.
• The project through design, construction, Commissioning
• The ongoing change that will inevitably occur in both the process and the equipment and engineering aspects.
Procedures:
• These cover engineering standards used in the project design, through to
commissioning phases and the facilities standard procedures (SOPs).
Documentation:
This section usually to identify the documentation that should be produced for the processing like
1. Engineering drawing
2. Equipment supplier drawing and documents
3. Factory acceptance document
4. IQ documents
5. OQ documents
6. PQ documents
Appendices:
• The appendices is mostly used VMP to hold the information of type of documents and formats that will be used in execution stage.
Calibration of pH Meter:
1. Calibration should be performed with at least two standard buffer solutions that span the range of pH values to be measured.
2. For general purposes buffers at pH 4 and pH 10 are acceptable.
• The pH meter has one control (calibrate) to set the meter, reading equal to the value of the first standard buffer
• A second control (slope) which is used to adjust the meter reading to the
value of the second buffer.
• A third control allows the temperature to be set.
Calibration of pH Meter Procedure:
• Turn on the pH meter on and wash with distilled water.
• Immerse the electrode in pH 4 buffer solution and press ‘standardize’ button after the reading has been stabilized
• Wash the electrode with distilled water and gently dry with tissue.
• Repeat the procedure for the pH 7 and pH 10 buffer solutions.
QUALIFICATION OF UV – VISIBLE SPECTROPHOTOMETER
UV-Visible spectroscopy is concerned with ultra violet and visible regions which ranges from 200-780nm.
QUALIFICATION:
• Qualification is an act or process to assure something complies with some
conditions, standard or specific requirements.
• Design Qualification: Documented evidence which shows that the plant design agrees with the design specifications of the customer.
• Installation Qualification: Written evidence is given that all parts of equipment are installed according to the equipment supplier’s and purchase specifications.
• Operational qualification: Documented evidence which shows that all parts of the plant and equipment work within parameters.
• Performance Qualification: Provides documented evidence that all parts of a plant and other processes produce products of specified quality under conditions of normal production for a longer period of time.
PERFORMANCE QUALIFICATION
• Wavelength accuracy
It is defined as the deviation of the wavelength reading at an absorption band and emission band from the wavelength of the band.
Acceptance: ± nm in UV range (200-380 nm) and
± nm in visible range (380-800 nm)
Three repeated scan of the same peak should be with in ±0.5 nm
• Stray light
Stray light is defined as the detected light of any wavelength that is out side the
hand width of the wavelength selected.
Acceptance: the transmittance of the solution in a 1cm cell Should be less than 0.01 or the absorbance value should be greater than 2
• Resolution power
The resolution of the UV-VIS spectrometer is related to its spectral hand width.
√ The smaller the band width the finer the resolution. The SBW depends on the shit width and the dispersive power of the monochromator.
Acceptance: The ratio of the absorbance at 269 nm and absorbance at 266 nm
should be greater than 1.5.
• Noise
Noise is the measurement affects the accuracy at the both end of the absorbance scale. Photon noise from the light source affects the accuracy of the measurement leads to low absorbance.
Acceptance: The RMS noise should be less than 0.001 AU
• Baseline flatness
The flat baseline test demonstrates that the ability of the instrument to normalise
the light intensity measurement and the spectral output at different wavelength
throughout the spectral range.
Acceptance: The measurement is typically less than 0.01 AU
• Stability
The lamp intensity is a function of the lamp age, temperature fluctuation and wavelength of the measurement. These changes can lead to errors in the value of the measurements, over an extended period of time.
Acceptance: The deflection is less than 0.002AU/ hr
• Photometric accuracy
Photometric accuracy is determined by comparing the difference between the measured absorbance of the reference material and the established value.
Acceptance: Six replicate measurements of the 0.006% w/v of the potassium dichromate solution at 235, 257, 313 and 350 nm should be less than 0.5% RSD.
• Linearity
The linear dynamic range of measurement is limited by stray light at high absorbance and by noise at low absorbance. The accuracy of the quantification of the sample depends on the precision and linearity of the Measurements.
Acceptance: Correlation coefficient r ≥ 0.999
GENERAL PRINCIPLES OF ANALYTICAL METHOD VALIDATION
• Method validation is the process used to confirm that the analytical procedure
employed for a specific test is suitable for its intended use.
• Results from method validation can be used to Judge the quality, reliability and consistency of analytical results.
• It is an integral part of any good analytical practice.
• As per FDA-guidelines
• Validation is establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its pre-determined specifications and quality attributes.
• Guideline presents information on the characteristics to be considered.
• Manufacturers to demonstrate -analytical procedure is suitable for its intended purpose.
• All analytical methods should be validated – whether
they indicate stability or not.
• Validated by R&D before being transferred to the quality control unit when appropriate
• All analytical methods intended to be used for analysing any clinical samples will need to be validated.