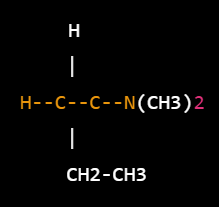Structure –
Phenylephrine is a medication used primarily as a decongestant, and it belongs to the class of drugs known as alpha-adrenergic agonists. Its chemical structure is as follows:
The molecule consists of a benzene ring (represented by the “H” on the top) attached to a carbon atom, which is in turn connected to a nitrogen atom with two methyl groups attached. The carbon atom is also connected to a chain of two carbon atoms and one hydrogen atom (represented by “CH2-CH3”), which serves as a substituent on the nitrogen atom. The entire molecule is hydrophobic, meaning it does not dissolve in water but can dissolve in organic solvents.
Synthesis –
it can be synthesized through a multistep process starting from benzene. The general synthetic route involves the following steps:
- Nitration: Benzene is first nitrated to produce nitrobenzene using a mixture of concentrated nitric acid and concentrated sulfuric acid.
- Reduction: Nitrobenzene is reduced to produce phenylhydroxylamine using a reducing agent such as iron filings and hydrochloric acid.
- Acylation: Phenylhydroxylamine is then acylated using acetic anhydride to form N-acetylphenylhydroxylamine.
- Alkylation: N-acetylphenylhydroxylamine is alkylated with 2-bromo-1-phenylethanol in the presence of a base such as potassium carbonate to produce phenylephrine.
HO H
| |
H3C–N–CH2–CH2–C–H
| |
Ph OH
The overall reaction can be summarized as:
Benzene → Nitrobenzene → Phenylhydroxylamine → N-Acetylphenylhydroxylamine → Phenylephrine
It’s worth noting that there are variations of this synthetic route that may use different reagents or reaction conditions to achieve similar results. Additionally, this process is typically carried out on an industrial scale and should not be attempted by individuals without proper training and equipment.
SAR ( structure-activity relationship) –
The SAR (structure-activity relationship) of it involves the modification of its chemical structure to optimize its pharmacological properties. Some modifications that have been made to the it structure include:
- Introduction of a hydroxyl group: This modification leads to the formation of hydrochloride salts of phenylephrine, such as phenylephrine hydrochloride. The hydroxyl group increases the polarity of the molecule, making it more water-soluble and improving its bioavailability.
- Substitution of the amine group: This modification has been used to develop more selective alpha-1 adrenergic receptor agonists, such as xylometazoline and oxymetazoline. These compounds have a higher affinity for alpha-1 adrenergic receptors than phenylephrine and are more effective as nasal decongestants.
- Addition of a bulky group: The addition of bulky groups to the phenylephrine molecule can increase its selectivity for alpha-1 adrenergic receptors, as well as its duration of action. For example, the addition of a tert-butyl group to phenylephrine results in the development of metizoline, which has a longer duration of action than phenylephrine.
- Alteration of the substituent on the benzene ring: The substituent on the benzene ring can be altered to modify the pharmacological properties of phenylephrine. For example, the replacement of the hydroxyl group on the benzene ring with a chlorine atom results in the development of chlorpheniramine, which has antihistaminic properties in addition to its decongestant activity.
Overall, the SAR of phenylephrine highlights the importance of optimizing the chemical structure of a drug to improve its pharmacological properties, such as its selectivity, duration of action, and bioavailability.
Mechanism –
it is a selective alpha-1 adrenergic receptor agonist, meaning it binds and activates the alpha-1 adrenergic receptors found in blood vessels, causing vasoconstriction. The mechanism of phenylephrine involves a series of steps, as follows:
- Binding to alpha-1 adrenergic receptors:it binds selectively to alpha-1 adrenergic receptors located on smooth muscle cells of blood vessels.
- Activation of G protein-coupled receptors: Upon binding to the alpha-1 adrenergic receptor, it activates a G protein-coupled receptor signaling pathway.
- Activation of phospholipase C: The activated G protein activates phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG).
- Release of intracellular calcium: IP3 binds to its receptor on the endoplasmic reticulum (ER), causing the release of intracellular calcium ions (Ca2+) into the cytoplasm.
- Activation of myosin light chain kinase: The increased cytoplasmic Ca2+ binds to and activates myosin light chain kinase (MLCK), which phosphorylates myosin light chains (MLCs).
- Contraction of smooth muscle cells: The phosphorylation of MLCs leads to the activation of myosin ATPase, which hydrolyzes ATP to produce energy for muscle contraction. This results in the contraction of smooth muscle cells and vasoconstriction.
Overall, phenylephrine’s mechanism involves the activation of G protein-coupled receptor signaling pathways and the subsequent release of intracellular calcium, leading to the contraction of smooth muscle cells and vasoconstriction.
Uses –
it has a variety of medical uses, including:
- Nasal decongestant: it is commonly used as a nasal decongestant to relieve congestion caused by allergies or the common cold. It works by constricting blood vessels in the nasal passages, reducing swelling and allowing for easier breathing.
- Hypotension treatment: Phenylephrine can be used to treat hypotension (low blood pressure) in certain medical conditions, such as septic shock or anesthesia-induced hypotension. It works by constricting blood vessels, which raises blood pressure.
- Ophthalmic solution: Phenylephrine is sometimes used as an ophthalmic solution to dilate the pupils during eye exams or procedures. It works by constricting the blood vessels in the eye, which allows for better visualization of the structures inside the eye.
- Topical vasoconstrictor: Phenylephrine is sometimes used as a topical vasoconstrictor in surgical procedures or to control bleeding in the nose or gums.
- Cardiac stress testing: Phenylephrine can be used in cardiac stress testing to increase heart rate and blood pressure, which helps to diagnose certain heart conditions.
It’s important to note that phenylephrine should only be used as directed by a healthcare professional and may have potential side effects, including increased blood pressure, anxiety, and cardiovascular effects.